Primary pollutants are any type of pollutant directly emitted into the environment from sources such as cars, power plants, and natural forest fires. They differ from secondary pollutants, which are formed in the atmosphere by chemical reactions involving primary pollutants. Primary pollutants contribute to the formation of secondary pollutants, which are harder to control due to their different synthesis pathways and limited understanding of their formation. One major secondary pollutant, ground-level ozone, is formed through the interaction of volatile organic compounds (VOCs) and nitrous oxides (NOx) with sunlight and heat. This, in turn, leads to the creation of photochemical smog, which is a significant issue in densely populated cities.
What You'll Learn

Primary pollutants from vehicles and power plants
Transportation and industrial activity are major sources of air pollution, with vehicles and power plants emitting a range of harmful primary pollutants. These pollutants are formed and released directly from specific sources, including cars, trucks, and buses, and industrial processes.
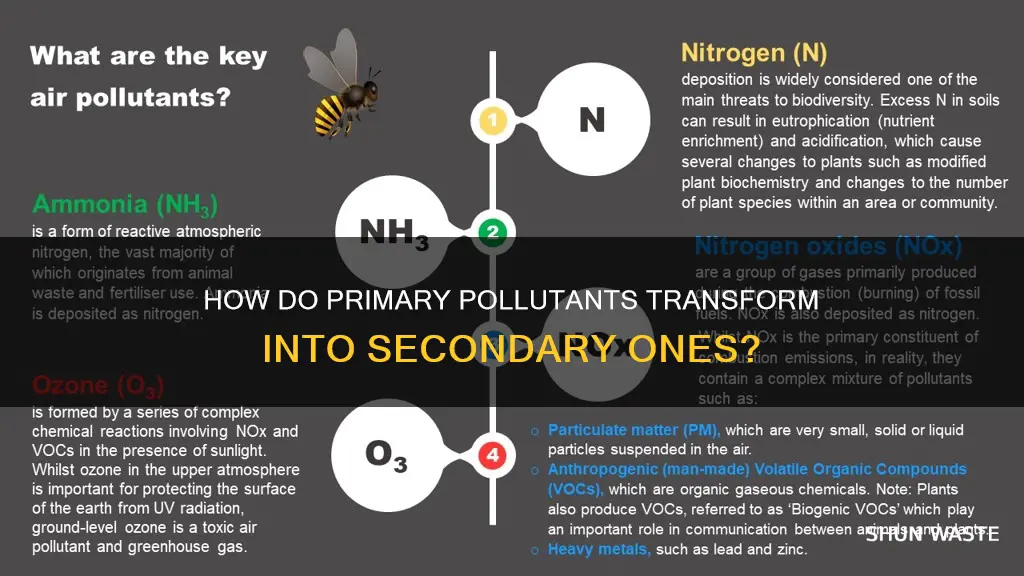
One of the primary pollutants emitted by vehicles and power plants is nitrogen oxides (NOx). NOx is produced during the combustion of fossil fuels, particularly in the burning of gasoline and diesel. NOx can irritate the lungs, weaken defences against respiratory infections, and contribute to the formation of ground-level ozone and particulate matter.
Carbon monoxide (CO) is another primary pollutant released by vehicles. It is a colourless, odourless, and poisonous gas formed by the combustion of fossil fuels. CO is dangerous as it blocks oxygen from reaching vital organs like the brain and heart. Vehicles, especially those powered by diesel and coal, also emit sulfur dioxide (SO2), which is formed by burning sulfur-containing fuels. SO2 poses significant health risks, especially to young children and asthmatics.
In addition to these, vehicles also release a range of toxic compounds, including volatile organic compounds (VOCs) and hydrocarbons. VOCs, such as benzene, acetaldehyde, and 1,3-butadiene, are linked to various types of cancer and cardiovascular issues. Hydrocarbons contribute to the formation of secondary pollutants like ozone, which is a powerful oxidant that can irritate the respiratory system and damage plant life.
The impact of these primary pollutants is significant, with exposure causing a range of adverse health effects and contributing to climate change. Efforts to reduce emissions, such as the development of clean vehicle and fuel technologies, are crucial to mitigate these impacts and improve air quality.
Namibia's Water Pollution: Business Impact and Responsibility
You may want to see also

Reactions with sunlight and heat
Primary pollutants are emitted directly from particular sources, such as vehicles, power plants, industrial processes, and natural sources. These can include particulates, carbon monoxide, nitrogen oxide, and sulfur oxide.
When primary pollutants react with sunlight and heat, they can undergo further reactions in the atmosphere to produce secondary pollutants. This process is known as photochemical smog formation, and it occurs when pollutants from automobile exhausts or industrial activities interact with sunlight and heat. This typically happens in cities with warm, dense atmospheres, where the inversion layers in the atmosphere trap primary pollutants, leading to the formation of smog.
One example of a secondary pollutant formed through this process is ozone. Ozone is a secondary pollutant found mainly in cities, created when nitrogen oxides (NOx) and volatile organic compounds (VOCs) react in the presence of sunlight. This reaction results in the formation of yellow clouds that are harmful to humans.
Another secondary pollutant influenced by sunlight and heat is acid rain. Acid rain is formed when sulfur dioxide, a primary pollutant emitted from industrial processes and power plants, reacts with oxygen and water vapour in the atmosphere to produce sulfuric acid. This process is enhanced by the presence of sunlight, which increases the rate of the chemical reaction.
Additionally, aerosols, which are fine droplets of pollutants, can reflect sunlight back into space, influencing the Earth's energy balance and cooling the atmosphere and the planet's surface. The formation of aerosols from primary pollutants can thus have a net cooling effect on the planet, impacting climate patterns.
Understanding IR Pollution: Causes and Origins
You may want to see also

Photochemical smog formation
Photochemical smog is a type of air pollution caused by the reaction of solar radiation with airborne pollutants. These pollutants include nitrogen oxides (NOx) and volatile organic compounds (VOCs) such as hydrocarbons. The nitrogen oxides are produced by car engines and emitted into the atmosphere, where they may react with sunlight to produce singular oxygen atoms. These atoms then combine with molecular oxygen to form ozone.
The formation of photochemical smog is influenced by several factors. Firstly, there must be a substantial source of primary pollutants, such as vehicular traffic, to emit sufficient NOx, hydrocarbons, and other VOCs into the air. Secondly, weather conditions play a crucial role. Warmer temperatures, ample sunlight, and calm air conditions with light winds can contribute to the formation of photochemical smog by inhibiting the dispersal of pollutants.
The presence of VOCs and NOx is essential for the formation of photochemical smog. When these pollutants interact with sunlight in the presence of heat, they react to form ground-level ozone. This ozone is a major component of photochemical smog and is considered the most toxic constituent, causing damage to agricultural and native plants.
In addition to ozone, other undesirable components of photochemical smog include nitrogen dioxide (NO2), peroxyacetyl nitrate (PAN), and aldehydes. These chemicals can cause eye irritation, respiratory issues, and plant damage if their concentrations are sufficiently high. The accumulation of these pollutants can result in reduced visibility and adverse health effects for people living in affected areas, particularly in densely populated cities.
Photochemical smog is a significant concern in large cities with a high number of motor vehicles and industrial activities, especially those located in basins or areas with complex terrain that hinders the dispersal of pollutants. Examples of cities prone to photochemical smog include Los Angeles, Mexico City, and Santiago.
Understanding Air Pollution: Primary Sources and Their Causes
You may want to see also

Health and environmental effects
Primary pollutants are pollutants that are emitted directly from a combustion process, such as carbon monoxide, nitrogen oxide, and sulfur oxide. While carbon dioxide is not directly harmful to human health, it is a greenhouse gas that contributes to climate change. On the other hand, carbon monoxide can be poisonous at high levels of exposure.
The health and environmental effects of primary pollutants are significant. High concentrations of SO2 can cause temporary breathing impairment in adults and children with asthma who are active outdoors. Individuals with asthma may also experience breathing difficulties, wheezing, chest tightness, or shortness of breath. Subgroups of the population that are particularly vulnerable to the effects of SO2 include individuals with heart or lung disease, the elderly, and children.
Primary pollutants also have a significant impact on the environment. For example, the emission of carbon dioxide (CO2) and other greenhouse gases contributes to climate change. The excessive emission of CO2 leads to an increase in the Earth's atmospheric temperature, causing global warming.
In addition to the direct health effects of primary pollutants, there are also indirect consequences. Environmental pollution caused by primary pollutants can lead to the formation of secondary pollutants, which are harder to control and can cause problems like photochemical smog. Fine fraction particles (PM2.5) and coarse fraction particles (PM10-2.5) are examples of secondary pollutants that contribute to air pollution and have adverse health effects.
The health effects of primary and secondary pollutants are diverse and widespread. Exposure to environmental pollution is a significant source of health risks worldwide, affecting respiratory, reproductive, neural, and cardiovascular systems, as well as contributing to the development of cancer. Industrialization, vehicle emissions, and contaminated soil and water are some of the main sources of pollutants that harm human health.
Overall, the health and environmental effects of primary pollutants are extensive and far-reaching. While some effects are immediate and direct, such as the impact of high SO2 concentrations on asthmatics, others are long-term and contribute to global issues like climate change and increased morbidity and mortality rates. Understanding and mitigating the impact of primary pollutants on human health and the environment is crucial for safeguarding the well-being of current and future generations.
Heavy Metal Pollution: Understanding Its Main Causes
You may want to see also

Regulatory changes and economic shifts
The transformation of primary pollutants into secondary pollutants is influenced by regulatory changes and economic shifts that impact emission sources and atmospheric conditions. Here are some paragraphs that discuss this in detail:
Regulatory Changes and Their Impact:
Regulatory interventions have played a significant role in reducing primary pollutant emissions. For example, the implementation of improved regulations and technological advancements have contributed to a notable decrease in primary pollutant emissions in recent years. These regulations often target specific sectors, such as the industrial and transportation sectors, which are major contributors to primary pollutant emissions. By enforcing stricter emission standards and promoting cleaner technologies, regulatory changes can directly influence the formation of secondary pollutants.
Economic Shifts and Emissions Sources:
Economic shifts, such as the transition from fossil fuel-based industries to renewable energy sources, can also impact the transformation of primary pollutants into secondary pollutants. For instance, a shift towards renewable energy sources like solar, wind, and hydropower can reduce the emissions from coal-fired power plants, natural gas power plants, and other fossil fuel-based industries. This, in turn, decreases the presence of primary pollutants in the atmosphere, disrupting the formation of secondary pollutants.
International Cooperation and Standards:
Addressing the challenges posed by primary and secondary pollutants often requires international cooperation and the establishment of global standards. Transboundary pollution, where pollutants travel across national borders, necessitates collaborative efforts between countries to implement effective regulatory measures. International agreements, such as the Paris Climate Agreement, play a crucial role in setting emission reduction targets and promoting sustainable practices on a global scale. These agreements influence the regulatory landscape and economic decisions, ultimately impacting the transformation of primary pollutants into secondary pollutants.
Impact of Economic Shifts on Atmospheric Conditions:
Economic shifts can also indirectly influence atmospheric conditions, thereby affecting the formation of secondary pollutants. For example, a shift towards more sustainable and environmentally friendly practices in various industries can lead to a reduction in the emission of primary pollutants. This, in turn, provides favourable conditions for the synthesis of secondary pollutants, which are formed through complex chemical reactions in the atmosphere.
Local Regulatory Efforts:
In addition to international agreements, local and regional regulatory efforts are crucial in mitigating primary and secondary pollutant formation. For instance, London, a city historically challenged by poor air quality, has implemented a dense and advanced regulatory monitoring network. This network comprises over 140 regulatory-grade automatic stations that monitor nitrogen dioxide (NO2) and particulate matter (PM10) levels, helping to identify hotspots and guide targeted interventions to improve air quality.
The Dark Side of Ocean Thermal Energy Conversion
You may want to see also
Frequently asked questions
Primary pollutants are pollutants that are emitted directly into the environment from particular sources. They can be emitted from cars, coal-fired power plants, natural gas power plants, biomass burning, natural forest fires, and volcanoes, among other sources.
Secondary pollutants are pollutants that form in the atmosphere as a result of reactions between primary pollutants. They are typically found downwind of primary emissions.
Primary pollutants interact with other chemicals in the atmosphere to form secondary pollutants. For example, volatile organic compounds (VOCs) and nitrous oxides (NOx) react with sunlight and heat to form ground-level ozone, a major secondary pollutant.
Examples of secondary pollutants include ground-level ozone, haze (secondary organic aerosol), and photochemical smog, which is formed from interactions between particulates, nitrogen oxides, ozone, and other air pollutants.



















