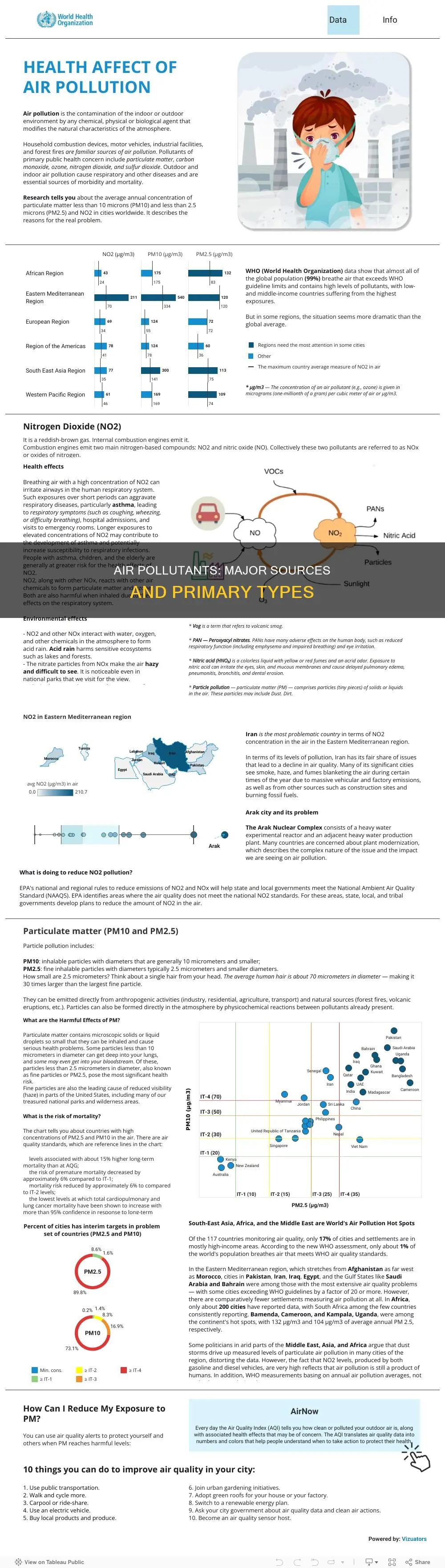
Air pollution is one of the most severe threats to humanity, and it is important to understand the major primary air pollutants and their sources to address this issue effectively. Primary air pollutants are those that are directly emitted from their source, and they can have highly detrimental effects on human health and the environment. These pollutants come from various sources, including transportation, industrial activities, energy generation, and agricultural processes. Some of the major primary air pollutants include carbon monoxide, nitric oxide, nitrogen dioxide, ozone, particulate matter, sulfur dioxide, volatile organic compounds, and ammonia. These pollutants are released into the atmosphere through vehicle exhausts, combustion processes, industrial operations, and agricultural practices, among other sources. Understanding the sources of these primary air pollutants is crucial for developing strategies to reduce emissions and improve air quality.
| Characteristics | Values |
|---|---|
| Major primary air pollutants | Carbon monoxide (CO), ammonia (NH3), nitric oxide (NO), nitrogen dioxide (NO2), ozone (O3), particulate matter (PM), sulphur dioxide (SO2), volatile organic compounds (VOCs), nitrogen oxides (NOx), black carbon (BC), polycyclic aromatic hydrocarbons (PAHs) |
| Sources of carbon monoxide | Vehicles, combustion engines, boilers, fireplaces, ovens, cooker hoods, central vacuum systems, tobacco smoke, propane heaters, power plants, biomass burning, forest fires, wood industry |
| Sources of ammonia | Agricultural processes, particularly fertilizer production and livestock waste management, cigarette smoke, cleaning solutions, three-way catalytic converters in urban areas |
| Sources of nitric oxide | Combustion of coal and petroleum |
| Sources of nitrogen dioxide | High-temperature combustion processes, such as automobile engines and industrial operations |
| Sources of sulphur dioxide | Industrial operations |
| Sources of volatile organic compounds | Photochemical reactions of other pollutants, such as nitrogen oxides and carbon monoxide, strong sunlight and UV radiation, electric motors in household appliances |
| Sources of nitrogen oxides | Combustion sources, vehicle exhausts, industrial activities, energy generation, agricultural processes |
| Sources of black carbon | Diesel and outdated motor vehicle engines, construction activities |
| Sources of polycyclic aromatic hydrocarbons | Incomplete burning of organic materials and substances |
| Sources of particulate matter | Power plants, vehicle traffic, construction sites, indoor stoves and heaters, industrial processes, agricultural and livestock farming, brake and tire abrasion from vehicles |
What You'll Learn
- Nitrogen oxides, carbon monoxide, volatile organic compounds, and sunlight cause ground-level ozone
- Nitrogen dioxide is formed during high-temperature combustion in engines and industrial operations
- Sulphur dioxide and nitrogen oxides cause acid rain, which is damaging to plant life
- Carbon monoxide is a colourless, odourless, toxic gas emitted by vehicles, combustion engines, and power plants
- Ammonia is a primary pollutant, largely emitted by agricultural practices and mobile sources

Nitrogen oxides, carbon monoxide, volatile organic compounds, and sunlight cause ground-level ozone
Ground-level ozone is a major air pollutant that is harmful to human health and the environment. Unlike stratospheric ozone, which is "good" as it protects against ultraviolet radiation from the sun, ground-level ozone is "bad" and can trigger a variety of health problems, especially for vulnerable individuals such as children, the elderly, and people with lung diseases like asthma.
Ground-level ozone is not emitted directly but is formed through chemical reactions involving nitrogen oxides (NOx), volatile organic compounds (VOCs), and sunlight. These precursor gases react in the presence of sunlight to produce ozone smog. NOx is primarily produced when fossil fuels, such as gasoline, oil, or coal, are burned in power plants, motor vehicles, and industrial processes involving high-heat combustion. Sources of VOCs include common consumer products like paint, household chemicals, motor vehicles, chemical plants, refineries, factories, and gas stations.
Nitrogen oxides are a family of pollutants that have significant impacts on both human health and the environment. They are formed through the combustion of fossil fuels and contribute to the formation of ground-level ozone. Nitrogen dioxide (NO2), a common nitrogen oxide, is known to lead to adverse respiratory effects, including airway inflammation and asthma, particularly for those living near major roadways. Additionally, nitrogen oxides can react with other compounds to form fine particulate matter (PM2.5), which poses even greater health risks as it can penetrate deeper into the lungs and bloodstream.
Carbon monoxide (CO) is another major primary air pollutant. It is a colourless, odourless, and tasteless toxic gas emitted directly from vehicles and combustion engines, as well as indoor sources such as boilers, fireplaces, and tobacco smoke. When inhaled, carbon monoxide inhibits the body's ability to carry oxygen to organs and tissues, posing a significant risk of death, especially for vulnerable individuals.
The presence of sunlight plays a crucial role in the formation of ground-level ozone. When NOx and VOCs react in the atmosphere under the influence of sunlight, they undergo photochemical reactions to produce ozone. This is why ground-level ozone levels tend to be higher during hot and sunny weather, particularly in urban environments. However, it's important to note that wind can transport ozone over long distances, leading to high ozone levels even in rural areas.
Air Quality Index: Pollutants That Affect Our Air
You may want to see also

Nitrogen dioxide is formed during high-temperature combustion in engines and industrial operations
Nitrogen dioxide (NO2) is one of the several oxides of nitrogen and a major air pollutant. It is a reddish-brown gas with a pungent, acrid odour. Nitrogen dioxide is formed during high-temperature combustion in engines and industrial operations. This process involves the reaction of nitrogen and oxygen gases in the air, particularly at high temperatures above 1300 °C (2600 °F).
In combustion engines, such as those in cars, the reaction between nitrogen and oxygen during fuel combustion produces nitrogen oxides, including nitrogen dioxide. This is especially prevalent in areas with high motor vehicle traffic, such as large cities, where nitrogen oxides emitted from car engines contribute significantly to air pollution.
Industrial operations, including the combustion of fossil fuels, are another significant source of nitrogen dioxide formation. Fossil fuel combustion from stationary sources accounts for 24% of man-made emissions, while mobile sources contribute 49%. The combustion of certain fuels with high nitrogen content, such as coal and oil, releases bound nitrogen as a free radical, which ultimately forms nitrogen dioxide (NO2).
The formation of nitrogen dioxide through high-temperature combustion is highly temperature-dependent. At high temperatures, molecular nitrogen (N2) and oxygen (O2) dissociate into their atomic states and participate in reactions that lead to the formation of nitrogen oxides. The rate of NOx formation increases with higher temperatures and longer residence times of nitrogen at those elevated temperatures.
To mitigate the formation of nitrogen dioxide and reduce emissions, various technologies have been implemented. For example, low-temperature combustion (LTC) technology can help reduce the thermal formation of NOx during combustion, although it may come at the cost of increased PM or soot production. Selective catalytic reduction (SCR) and selective non-catalytic reduction (SNCR) are also effective in reducing NOx emissions by converting exhaust gases into nitrogen and water.
Livestock's Role in Air Pollution: A Comprehensive Overview
You may want to see also

Sulphur dioxide and nitrogen oxides cause acid rain, which is damaging to plant life
Major air pollutants include carbon monoxide (CO), ammonia (NH3), nitric oxide (NO), nitrogen dioxide (NO2), ozone (O3), particulate matter (PM), sulphur dioxide (SO2), and volatile organic compounds (VOCs). Sulphur dioxide and nitrogen oxides are two such pollutants that cause acid rain, which has a detrimental impact on plant life.
Acid rain is caused when sulphur dioxide and nitrogen oxides are emitted into the atmosphere and transported by wind and air currents. These pollutants react with water, oxygen, and other chemicals to form sulphuric and nitric acids, which then mix with water and other materials before falling to the ground. While volcanoes and rotting vegetation are natural sources of these chemicals, most acid rain is a product of human activities, particularly the burning of fossil fuels to generate electricity.
The effects of acid rain are widespread and damaging to the environment, including plant life. Acid rain has a low pH, making it more acidic than normal rain. When acid rain reaches the Earth, it flows across the surface, enters water systems, and seeps into the soil. This process weakens trees and plants by dissolving nutrients in the soil before they can be absorbed. A notable example is the virtual tree graveyard of Norway spruce in Poland, which bears the scars of acid rain damage.
In addition to its impact on plant life, acid rain also affects physical structures, such as limestone buildings and cars. When it occurs as inhalable fog, acid rain can cause health issues in humans, including eye irritation and asthma.
To combat the detrimental effects of acid rain, it is essential to reduce the release of its pollutant sources. This involves burning fewer fossil fuels and implementing air quality standards to regulate emissions. The Clean Air Act of 1990 in the United States is an example of such regulation, which has helped reduce sulphur dioxide emissions and contributed to the recovery of some ecosystems affected by acid rain.
Surgical Masks: Effective Air Pollution Protection?
You may want to see also

Carbon monoxide is a colourless, odourless, toxic gas emitted by vehicles, combustion engines, and power plants
Carbon monoxide is a significant primary air pollutant. It is a colourless, odourless, and toxic gas that poses a serious threat to human health and the environment. The danger of carbon monoxide lies in its ability to inhibit the body's oxygen transport system, which can lead to fatal consequences, especially for vulnerable individuals such as infants, the elderly, and those with heart and respiratory conditions.
Sources of Carbon Monoxide:
- Vehicle Emissions: One of the major sources of carbon monoxide is vehicle exhaust from cars, buses, taxis, and other road transport. The combustion of fossil fuels, especially diesel and outdated motor vehicle engines, contributes significantly to carbon monoxide emissions.
- Combustion Engines: Combustion engines, including those used in vehicles and industrial operations, produce carbon monoxide. High-temperature combustion processes, particularly in automobile engines, are a significant source of this pollutant.
- Power Plants: Carbon monoxide is emitted from power plants, contributing to outdoor air pollution.
- Indoor Sources: Carbon monoxide is also generated indoors from boilers, fireplaces, ovens, cooker hoods, tobacco smoke, and propane heaters. These sources can lead to dangerous levels of carbon monoxide indoors, especially in poorly ventilated spaces.
- Biomass Burning and Forest Fires: The burning of biomass, including wood and other organic materials, releases carbon monoxide into the atmosphere. Forest fires, whether natural or human-induced, also contribute to carbon monoxide emissions.
- Wood Industry: The wood industry, through various processes, can emit carbon monoxide. This includes the use of combustion engines and industrial activities.
It is important to note that carbon monoxide is not the only air pollutant produced by these sources. Other harmful pollutants, such as nitrogen oxides (NOx), particulate matter (PM), and volatile organic compounds (VOCs), are often emitted alongside carbon monoxide. These pollutants can have detrimental effects on human health and the environment, contributing to respiratory issues, lung function decline, and ecological damage.
To mitigate the impact of carbon monoxide and other air pollutants, it is crucial to implement measures such as improving fuel efficiency, adopting cleaner technologies, enforcing stricter emission controls, and promoting sustainable practices in industries, transportation, and energy generation sectors. Additionally, the development of policies and investments that support cleaner transport, energy-efficient industries, and optimized urban development can help reduce carbon monoxide emissions and improve air quality.
Air Quality Alert: Code Orange Explained
You may want to see also

Ammonia is a primary pollutant, largely emitted by agricultural practices and mobile sources
Air pollution is a pressing issue that poses significant risks to human health, the environment, and property. Ammonia (NH3) is a primary pollutant and a major contributor to air pollution, particularly in the form of fine particulate matter (PM2.5). This colourless gas has a pungent odour and is known to irritate the eyes, nose, throat, and respiratory tract, even in small amounts. Inhalation of ammonia can lead to reduced lung function, throat irritation, and increased coughing and phlegm production.
Agricultural practices are the dominant source of ammonia emissions on a national scale. Livestock waste, manure spreading, and the use of synthetic fertilizers are key contributors to these emissions. Agriculture accounts for over 81% of global ammonia emissions, significantly impacting air quality and human health. The direct impact of ammonia on individuals handling livestock has been observed, including respiratory health effects such as reduced lung function and irritation to the throat, eyes, and respiratory tract.
In addition to agricultural sources, mobile sources, particularly in urban areas, are also responsible for ammonia emissions. Three-way catalytic converters in vehicles can over-reduce NOx emissions, leading to the release of ammonia. This is a notable concern in urban settings, where ammonia emissions from traffic contribute to air pollution and its associated health risks.
Ammonia plays a significant role in the formation of secondary particulate matter (PM2.5). When combined with other pollutants in the atmosphere, ammonia contributes to the fine particulate matter that can penetrate deep into the lungs, causing long-term illnesses such as Chronic Obstructive Pulmonary Disease (COPD) and lung cancer. The economic impact of PM2.5-related health issues is substantial, resulting in billions of dollars in losses to the global economy each year.
Reducing ammonia emissions is crucial to mitigating the health and environmental risks associated with air pollution. By regulating and reducing NH3 emissions, particularly from agricultural practices and mobile sources, we can effectively decrease ambient PM2.5 levels, protect human health, and minimize the economic losses attributed to air pollution.
Plants' Resilience: Adapting to Air Pollution
You may want to see also
Frequently asked questions
Major primary air pollutants include carbon monoxide (CO), ammonia (NH3), nitric oxide (NO), nitrogen dioxide (NO2), ozone (O3), particulate matter (PM), sulphur dioxide (SO2) and volatile organic compounds (VOCs).
Carbon monoxide is emitted directly from vehicles and combustion engines. Indoors, sources of carbon monoxide include boilers, fireplaces, ovens, tobacco smoke, and propane heaters. Other sources include power plants, biomass burning, forest fires, and the wood industry.
Ammonia is emitted largely as a result of agricultural practices, including direct emissions from livestock waste, the spreading of manure, and the use of synthetic fertilizers. In urban areas, ammonia emissions are dominated by mobile-source emissions, which result from three-way catalytic converters over-reducing NOx to ammonia.
Nitrogen dioxide forms when nitrogen and oxygen gases react during high-temperature combustion processes, such as those found in automobile engines and industrial operations.
Sources of particulate matter include power plants, vehicle traffic, construction sites, indoor stoves and heaters, industrial processes, agricultural and livestock farming, and brake and tire abrasion from vehicles.







