Sulfate is a naturally occurring substance in drinking water. However, high levels of sulfate in drinking water have been associated with diarrhea, dehydration, and laxative effects in humans, particularly in infants and travelers. In animals, high levels of sulfate may lead to severe, chronic diarrhea and even death. Due to these health concerns, the U.S. Environmental Protection Agency (EPA) has included sulfate in the Drinking Water Contaminant Candidate List. This article will explore the topic of sulfate as a potential water pollutant, discussing its sources, treatment methods, and health effects.
| Characteristics | Values |
|---|---|
| Occurrence | Sulfate occurs naturally in drinking water and groundwater. |
| Health Effects | High levels of sulfate in drinking water have been associated with diarrhea, dehydration, and laxative effects. Sensitive groups such as infants and young children may be at greater risk. Animals, especially young animals, may also be sensitive to high sulfate levels, with potential severe health consequences. |
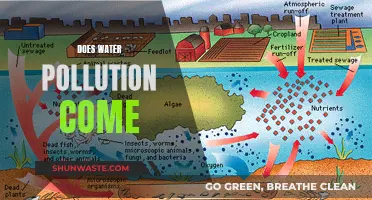
| Contamination Sources | Both natural sources and human activities can contribute to sulfate contamination in water. Natural sources include mineral dissolution, while human activities such as agricultural practices and industrial wastewater runoff are also significant contributors. |
| Treatment Methods | Various methods can be used to remove sulfate from water, including reverse osmosis, distillation, anion exchange, and adsorptive media filtration. |
| Regulatory Status | The U.S. Environmental Protection Agency (EPA) has included sulfate in the Drinking Water Contaminant Candidate List. The Safe Drinking Water Act (SDWA) directs the EPA and the Centers for Disease Control and Prevention (CDC) to study the health effects of sulfate exposure. However, it is currently unregulated, and the EPA is evaluating whether to implement regulations. |
What You'll Learn
- Sulfate occurs naturally in drinking water and can cause health issues such as diarrhoea
- Sulfate is a contaminant in groundwater, with health risks for humans and animals
- Sulfate pollution in air is caused by human-generated shipping emissions
- The US Environmental Protection Agency (EPA) regulates sulfate as a criteria pollutant
- Sulfate levels can be reduced in water through distillation, reverse osmosis, and anion exchange

Sulfate occurs naturally in drinking water and can cause health issues such as diarrhoea
Sulfate is a naturally occurring substance in drinking water. It is one of the 50 chemical and 10 microbiological contaminants/contaminant groups included on the Drinking Water Contaminant Candidate List published on March 2, 1998. The US Environmental Protection Agency (EPA) and the Centers for Disease Control and Prevention (CDC) conducted a study to establish a relationship between the adverse health effects of sulfate ingestion and the levels of sulfate in drinking water.
The study found that sulfate in drinking water at concentrations exceeding 500-700 mg/liter is a cause of diarrhea. However, in a controlled study with 10 healthy subjects, no subjects reported diarrhea or passed more than three stools per day. The subjects were given a constant diet and fluid intake, with the fluid consisting of 36 ml/kg/day of drinking water of various known sulfate concentrations and 500 ml of other fluid. In another study with 10 normal adult subjects, a concentration of 1200 mg/liter, which is higher than reported in US municipal water sources, caused a measurable but clinically insignificant increase in stool mass and a decrease in stool consistency and appearance time, but no change in stool frequency and no complaint of diarrhea.
Despite the conflicting findings, health concerns regarding sulfate in drinking water persist due to reports of diarrhoea in susceptible populations, such as infants and travellers, who are more sensitive to sulfate than adults. Diluting water with high sulfate levels can help prevent diarrhoea and dehydration in these vulnerable groups. It is recommended that water with a sulfate level lower than 500 milligrams per liter (mg/L) be used to make infant formula.
Sulfate levels in drinking water can vary depending on the region. For example, in Minnesota, the level of sulfate in most groundwater is low, less than 250 mg/L, while high levels of sulfate (sometimes above 1000 mg/L) are more common in the southwestern areas of the state and along its western boundary.
Water Pollution's Impact on Plant Growth and Health
You may want to see also

Sulfate is a contaminant in groundwater, with health risks for humans and animals
Sulfate is a contaminant that occurs naturally in groundwater. It is the most stable form of sulfur in water under aerobic conditions. While sulfate is not extremely toxic, it does pose health risks to humans and animals when ingested in high amounts.
In humans, the health effects of sulfate in drinking water have been studied by the U.S. Environmental Protection Agency (EPA) and the Centers for Disease Control and Prevention (CDC). Their research has found that high levels of sulfate in drinking water can cause osmotic diarrhea, especially in susceptible populations such as infants and travellers. This is due to the laxative effects of sulfate, which can also lead to dehydration. The Safe Drinking Water Act (SDWA) specifies a maximum contaminant level of 250 milligrams per liter (mg/L) for sulfate in drinking water, based on taste and odor considerations. However, this is not a federally enforceable standard, and it is estimated that about 3% of public drinking water systems in the U.S. may have sulfate levels above this threshold.
Animals are also sensitive to high levels of sulfate in their water sources. Young animals, in particular, can experience severe, chronic diarrhea and even death when exposed to high sulfate levels. Diluting water with high sulfate content or gradually introducing animals to water with high sulfate levels can help prevent these adverse effects.
The presence of sulfate in groundwater is influenced by both natural sources and human activities. Acid mine drainage, for example, is a significant source of sulfate contamination in groundwater. Various treatment methods are available to remove sulfate from drinking water, including reverse osmosis, distillation, and anion exchange. These methods can effectively reduce sulfate levels and mitigate the potential health risks associated with high sulfate concentrations in water.
How Sodium in Water Affects Pollution
You may want to see also

Sulfate pollution in air is caused by human-generated shipping emissions
Sulfate is a naturally occurring substance in drinking water. However, high levels of sulfate in drinking water can lead to adverse health effects, including osmotic diarrhoea, dehydration, and catharsis. These impacts are of particular concern for vulnerable groups, such as infants, who are more susceptible to the laxative effects of sulfate. According to the US Environmental Protection Agency (EPA), about 3% of public drinking water systems in the country may have sulfate levels of 250 milligrams per liter or higher.
While sulfate in water is a concern, this answer will focus on the topic of sulfate pollution in the air caused by human-generated shipping emissions. Shipping emissions have long been recognised as a significant contributor to air pollution, with ships burning sulfur-rich "bunker oil" and releasing sulfate particles into the atmosphere. These emissions have led to the formation of "ship tracks," polluted marine clouds that trail ocean-crossing vessels and leave a signature of modern trade in the sky.
Research has revealed that ships may be responsible for a much larger proportion of sulfate pollution in coastal areas than previously thought. A study in Southern California utilised a technique to identify the source of sulfate produced during combustion, finding that ships burning bunker oil contributed significantly to the fine particulate matter suspended in the region's coastal air. This discovery highlights the impact of shipping emissions on air quality and human health, as fine particles have been linked to an increased risk of respiratory illness and cardiopulmonary mortality.
To address this issue, fuel regulations have been implemented to reduce sulfur emissions from ships. In 2020, the International Maritime Organization (IMO) introduced a global standard requiring an 86% reduction in fuel sulfur content. This regulation has had a positive impact, as evidenced by a significant decrease in ship tracks observed via satellite. The reduction in sulfur emissions is expected to have a cooling effect on the climate system, as pollution increases the reflectivity of clouds, offsetting some of the warming caused by CO2.
However, it is important to note that the shift towards lower-sulfur shipping fuel has been described as "inadvertent geoengineering." While it may mitigate human-induced climate impacts and reduce health issues from air pollution, presenting it as an accidental solution could lead to misguided assumptions about future emission-curbing policies. Nevertheless, the implementation of fuel regulations demonstrates a step in the right direction towards reducing sulfate pollution in the air caused by human-generated shipping emissions.
Blue Herons: Water Polluters or Innocent Birds?
You may want to see also

The US Environmental Protection Agency (EPA) regulates sulfate as a criteria pollutant
Sulfate is a naturally occurring substance in drinking water. However, it is also a contaminant that can enter water sources through both natural sources and human activities. The presence of sulfate in water, especially at high levels, has been associated with adverse health effects, such as diarrhoea, dehydration, and laxative effects. These health concerns have prompted investigations and regulations by the US Environmental Protection Agency (EPA).
The EPA has recognised the potential health risks associated with sulfate in drinking water and has taken steps to address this issue. Under the Safe Drinking Water Act (SDWA) of 1996, the EPA, in collaboration with the Centers for Disease Control and Prevention (CDC), conducted a study to establish the relationship between sulfate consumption and health outcomes. The study, known as the "Sulfate Study," specifically examined the link between high sulfate levels in tap water and reports of osmotic diarrhoea in susceptible populations, including infants and transients."
While the EPA has not yet established specific regulations for sulfate in drinking water, they have included it on the Drinking Water Contaminant Candidate List. The SDWA directs the EPA to evaluate and determine the need for regulation of contaminants on this list. The EPA is in the process of reviewing various documents, public comments, and relevant studies to make an informed decision on whether to regulate sulfate as a National Primary Drinking Water Regulation (NPDWR).
The EPA has set a secondary maximum contaminant level (SMCL) of 250 milligrams per liter (mg/L) for sulfate in drinking water. This guideline, while not federally enforceable, serves as a recommendation for states and public water systems. The EPA estimates that approximately 3% of public drinking water systems in the country may have sulfate levels at or above this threshold. The agency continues to monitor and assess the presence of sulfate in water systems, considering various risk management factors and treatment technologies.
In summary, while the US Environmental Protection Agency (EPA) does not currently regulate sulfate as a primary contaminant, it acknowledges its potential health impacts and is actively evaluating the need for regulatory action. The EPA's ongoing efforts reflect its commitment to ensuring safe drinking water and protecting public health.
Water Quality: What's in Our Glasses?
You may want to see also

Sulfate levels can be reduced in water through distillation, reverse osmosis, and anion exchange
Sulfate is a naturally occurring substance in drinking water. However, high levels of sulfate in drinking water can lead to adverse health effects, particularly for vulnerable groups such as infants and young children. The consumption of water with high sulfate concentrations has been linked to osmotic diarrhea, dehydration, and laxative effects. To mitigate these health risks, it is crucial to reduce sulfate levels in water through effective treatment methods.
Distillation is one such method that involves boiling water to produce steam. As the steam rises, it leaves behind contaminants, including sulfate. Properly operated distillation units can eliminate nearly 100% of sulfate ions, making it a highly effective treatment option.
Reverse osmosis is another technique that forces water through a membrane with tiny pores. This membrane acts as a barrier, trapping contaminants like sulfate, while allowing water molecules to pass through. Reverse osmosis systems typically remove between 93% and 99% of sulfate, depending on the specific treatment unit.
Anion exchange is the most widely used method for treating large volumes of water for commercial, livestock, and public supplies. This process involves replacing negatively charged ions, such as sulfate, with sodium chloride or potassium chloride (salts). By substituting the sulfate ions with these salts, the water becomes suitable for various applications.
It is important to note that while these treatment methods are effective in reducing sulfate levels, proper operation and maintenance of water treatment systems are essential. Homeowners and water treatment professionals should follow the manufacturer's recommendations to ensure the optimal performance of these systems.
In summary, distillation, reverse osmosis, and anion exchange play crucial roles in reducing sulfate levels in water, thereby minimizing potential health risks associated with the consumption of water containing high sulfate concentrations. By employing these treatment methods, we can improve water quality and safeguard the well-being of vulnerable populations.
Human Ashes: Water Pollution and Environmental Impact
You may want to see also
Frequently asked questions
Sulfate is a substance that occurs naturally in drinking water. However, it can also be a contaminant in water bodies due to both natural sources and human activities. It is one of the 50 chemical contaminants on the Drinking Water Contaminant Candidate List.
High levels of sulfate in drinking water can cause dehydration, diarrhoea, and a laxative effect in humans, especially infants. It can also be harmful to animals. The U.S. Environmental Protection Agency (EPA) has set a secondary maximum contaminant level (SMCL) of 250 milligrams per liter (mg/L) for sulfate in drinking water.
Sulfate can be removed from water through various methods such as reverse osmosis, distillation, anion exchange, and adsorptive media filtration.