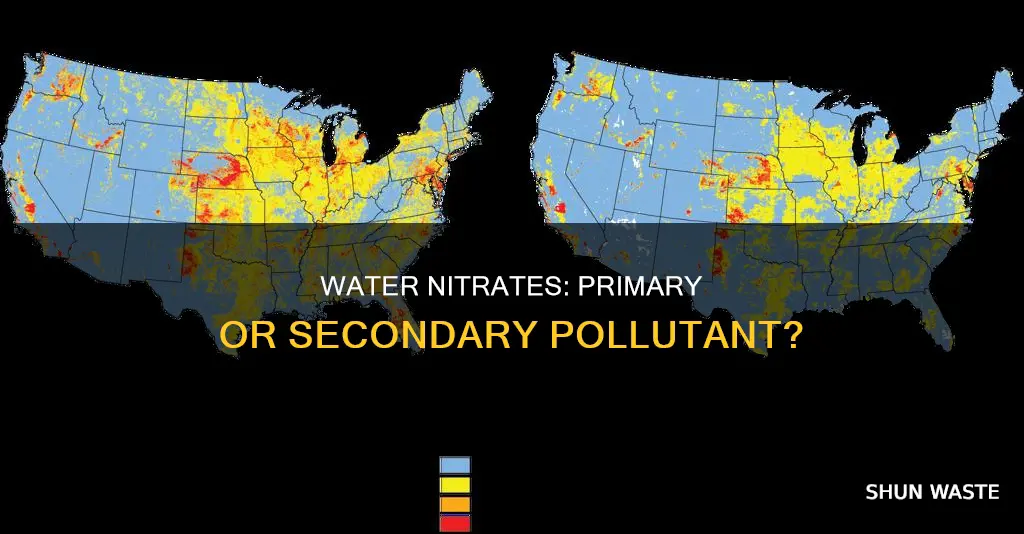
Nitrates are a growing concern for water quality, with their presence in water supplies posing a significant threat to human health. Nitrates are a common groundwater contaminant, and their high solubility and mobility make them challenging to manage. Sources of nitrates in water include agricultural runoff, septic systems, and natural processes, with human activities increasingly contributing to the problem. The impact of nitrates on drinking water has been linked to various health issues, particularly in infants and young children. As a result, nitrate contamination has become a critical issue for communities worldwide, with regulatory controls and local initiatives aiming to tackle this complex problem.
| Characteristics | Values |
|---|---|
| Type of Pollutant | Primary |
| Natural or Human-Made | Natural and Human-Made |
| Main Sources | Fertilizers, manure, septic systems, wastewater treatment plants, dairies, landfills, animal feedlots, animal waste, industrial and automobile emissions |
| Occurrence | Lakes, rivers, groundwater |
| Affected Areas | California, Minnesota, Sonqor plain in Iran, Europe, the US |
| Health Hazards | Methemoglobinemia, formation of nitrosamine compounds, esophageal cancer, gastric cancer, blue baby syndrome, thyroid problems, adverse pregnancy outcomes, cancers (particularly colorectal) |
| Regulatory Controls | Nitrates Directive in Europe, local efforts such as the Central Valley Water Board in California |
| Maximum Limits | 10 mg/L as per the U.S. Environmental Protection Agency (EPA), 50 mg/L as per the European Commission's Nitrates Directive |
What You'll Learn

Nitrates are a primary pollutant in water
Nitrates are a primary contaminant of groundwater and surface water. They can enter water systems through various sources, including agricultural practices, septic systems, wastewater treatment plants, and natural conditions. In agricultural contexts, nitrates are present in fertilizers and manure, which can leach into the soil and eventually contaminate groundwater. This is particularly common in areas with high nitrogen input, well-drained soils, and limited woodland areas relative to cropland.
The impact of human activities, such as excessive use of agricultural chemical fertilizers, substandard discharge of sewage, and landfill leakage, has led to an increase in nitrate concentration in freshwater annually. This has become a significant environmental concern, attracting the attention of scholars and regulatory bodies worldwide.
The presence of nitrates in drinking water can have adverse effects on human health. High nitrate levels can cause various health issues, including respiratory problems, reproductive issues, and harm to the kidney, spleen, and thyroid in both children and adults. Ingestion of nitrate-contaminated water by infants can lead to low oxygen levels in the blood, a potentially fatal condition. Additionally, studies have linked long-term exposure to nitrates in drinking water with thyroid problems, adverse pregnancy outcomes, and an increased risk of certain cancers.
To address nitrate pollution, regulatory measures such as the Nitrates Directive in Europe have been implemented. This directive aims to protect water quality by preventing nitrates from agricultural sources from polluting ground and surface waters and by promoting good farming practices. Local initiatives, such as programs focused on irrigated lands and dairy management, also play a role in mitigating nitrate pollution. Accurate identification of nitrate pollution sources is crucial for ensuring water safety and effectively controlling nitrate levels in freshwater environments.
Water and Life: Is There a Link?
You may want to see also

They are dangerous to human health, especially for infants
Nitrates are a secondary pollutant, entering water supplies as a result of agricultural activities. Nitrogen is a crucial nutrient that helps plants and crops grow, but high concentrations of nitrates are harmful to human health. Nitrates are dangerous to human health, especially for infants, as they can cause methemoglobinemia, also known as blue baby syndrome. This is a condition in which the blood's ability to carry oxygen is compromised, leading to shortness of breath and, if left untreated, death.
Infants are the most vulnerable to nitrate contamination, and it is recommended that parents test their well water before bringing their newborn baby home, especially if they plan to use formula made with water. Bottled water is advised in such cases until a safe level of nitrates in the water is assured. This is because infants under six months old who consume water with nitrate levels above the MCL (Maximum Contaminant Level) are at risk of serious illness and even death.
The regulatory limit for nitrate in public drinking water supplies is set to prevent infant methemoglobinemia, but other health effects have not been fully considered. Research has indicated a link between nitrate ingestion and an increased risk of specific cancers and birth defects, and central nervous system defects. Studies have also found a positive association between higher drinking water nitrate exposure during pregnancy and neural tube defects.
Populations with the highest exposure to nitrates in their drinking water are those living in agricultural regions, particularly those drinking water from shallow wells near nitrogen sources, such as crop fields and animal feeding operations. In the US, between 10 to 15 percent of public wells in California exceed the state's standards for nitrate, and similar issues have been found in Minnesota, where over 10 percent of private wells sampled in some townships had nitrate levels above 10 mg/L.
Water Pollution: Understanding the Sources and Their Impact
You may want to see also

Nitrates enter water through fertiliser, manure, and sewage
Nitrates are a growing problem in water supplies, and they enter water through various means, including fertiliser, manure, and sewage. Nitrogen is a crucial nutrient for plants and crops, but high concentrations are harmful to people and nature. Pure, clean water is vital to human health and natural ecosystems, and nitrate contamination poses a significant threat to both.
Fertilisers are a significant source of nitrates in water. Nitrogen fertilisers, in particular, are commonly used on farm crops and contain nitrates. These nitrates are highly soluble and can easily travel with water from the soil, leading to nitrate contamination in water supplies. The use of fertilisers in agriculture is a primary contributor to nitrate pollution in Europe. When used in excess, nitrogen from fertilisers can enter groundwater through leaching and reach surface water through runoff from agricultural fields.
Manure is another source of nitrates in water. The European Commission has recognised the impact of manure on water pollution, granting derogations to several countries, including the Netherlands, Ireland, and Denmark, to apply more than 170 kg/ha of nitrogen from manure. Manure contains nitrogen, which, when applied to fields, can leach into groundwater and run off into surface water, contributing to nitrate pollution.
Sewage is also a contributor to nitrates in water. Septic systems and wastewater treatment plants are identified as sources of nitrate contamination. Sewage discharge and wastewater treatment can introduce high levels of nitrates into water supplies, impacting both groundwater and surface water.
The presence of nitrates in water is considered a secondary pollutant. While nitrates occur naturally in soil and are necessary for plant growth, human activities, such as agriculture and wastewater treatment, have significantly increased their concentration in water supplies. These elevated levels of nitrates in water can have detrimental effects on human health and natural ecosystems, making it crucial to address nitrate pollution and implement effective regulatory controls.
Water Pollution's Impact on Global Warming
You may want to see also

Nitrate pollution is difficult and expensive to tackle
Nitrate pollution is a pressing issue, particularly in agricultural areas, and it is a challenge to address for several reasons. Firstly, nitrate contamination is often the result of human activities such as the use of fertilisers, septic systems, and animal waste. These sources can lead to high levels of nitrates in water bodies through runoff or leakage, which can be difficult to control and prevent.
One of the main challenges in tackling nitrate pollution is the cost associated with implementing effective measures. Nitrate removal from drinking water supplies, for example, can be expensive, especially for communities that rely on untreated groundwater and lack the necessary water treatment infrastructure. This is a significant issue, as seen in California, where between 10 and 15 percent of public wells exceed the state's nitrate standards and require treatment or blending with high-quality water.
Another difficulty in addressing nitrate pollution is the time and resources needed for monitoring and enforcement. Regulatory bodies are tasked with regularly testing water quality and identifying areas at risk of pollution, which can be a cumbersome and resource-intensive process. This is further complicated by the fact that nitrate pollution can come from various sources, including natural processes, making it harder to pinpoint and address specific sources effectively.
Additionally, nitrate pollution has cumulative and far-reaching impacts on ecosystems, which makes remediation complex. High nitrate levels in water bodies can lead to excessive algae growth, disrupting the natural balance and depleting oxygen levels in the water. This, in turn, affects aquatic life and the overall health of the ecosystem. Reversing or mitigating these effects requires a comprehensive and long-term approach, which can be challenging to develop and implement.
Furthermore, nitrate pollution is a persistent issue due to the nature of nitrates themselves. As nitrate is the oxidized form of dissolved nitrogen, it is highly mobile and can easily leach into the soil and water supply. This mobility makes it challenging to contain and remove nitrates from affected areas. In addition, while nitrates are naturally occurring and essential for plant growth, their excessive use in agriculture and subsequent runoff contribute significantly to water pollution.
Ways to Reduce Water Pollution and Save Our Oceans
You may want to see also

Some regulatory controls are in place, but more is needed
Nitrates are a significant concern for water quality, and while there are some regulatory controls in place, the general consensus is that a more comprehensive approach is needed to address this issue effectively. Nitrates are a primary pollutant, and their presence in water is mainly due to agricultural activities and the use of fertilisers and manure. This has led to nitrate contamination in surface water and groundwater, which poses a threat to both the environment and human health.
In recognition of the problem, the European Commission has implemented the Nitrates Directive, which aims to protect water quality across Europe. This directive focuses on preventing nitrate pollution from agricultural sources and promoting good farming practices. It establishes codes of good agricultural practice and measures to reduce water pollution. The directive also requires EU member states to monitor water quality and identify areas at risk of pollution, known as Nitrate Vulnerable Zones (NVZs). These areas are of particular concern due to their high levels of nitrates, which can be detrimental to aquatic ecosystems and human health.
In the United States, the Environmental Protection Agency (EPA) has established National Primary Drinking Water Regulations to control contaminants, including Cryptosporidium, Giardia lamblia, viruses, and Legionella. These regulations set standards for the maximum levels of disinfectants and contaminants allowed in drinking water to protect public health. However, the regulations primarily focus on microbial contaminants and do not specifically target nitrate pollution.
While these regulatory efforts are a step in the right direction, more needs to be done to address the complex issue of nitrate pollution effectively. The challenges lie in tackling the diverse sources of nitrate contamination and the varying levels of nitrate concentrations in different regions. For example, in California, nitrate contamination of groundwater is a significant issue, with up to 15% of public wells exceeding state standards. The treatment or blending of contaminated water is expensive, especially for systems relying on untreated groundwater without the necessary infrastructure.
To address these challenges, a multifaceted approach is required. This includes implementing stricter controls on agricultural practices, such as fertiliser usage and manure management, and providing support for communities to improve their water treatment infrastructure. Additionally, further research and studies are needed to comprehensively understand the health risks associated with nitrate ingestion, especially regarding cancer, adverse reproductive outcomes, and other health effects. By combining regulatory measures with scientific knowledge, we can develop more effective strategies to protect our water resources and safeguard public health from the harmful effects of nitrate pollution.
Flint, Michigan: A Tale of Polluted Water Crisis
You may want to see also
Frequently asked questions
Nitrate is the oxidized form of dissolved nitrogen and is the main source of nitrogen for plants. It occurs naturally in soil but can also be human-made, such as from nitrogen fertilizers. Nitrate can enter water through leaching and reach surface water through runoff from agricultural fields.
Nitrate in water can have various effects on human health, especially for infants and young children. Ingestion of nitrate in drinking water can cause low oxygen levels in the blood, a potentially fatal condition. It can also induce diseases such as methemoglobinemia, esophageal cancer, gastric cancer, and blue baby syndrome. In addition, high concentrations of nitrate in water can lead to excessive growth of algae, affecting the natural ecosystem and causing depletion of oxygen in the water.
Nitrate is considered a primary pollutant in water. It is one of the most common groundwater contaminants, and its presence in water supplies is a growing concern in many regions, including California, Minnesota, and Iran.







