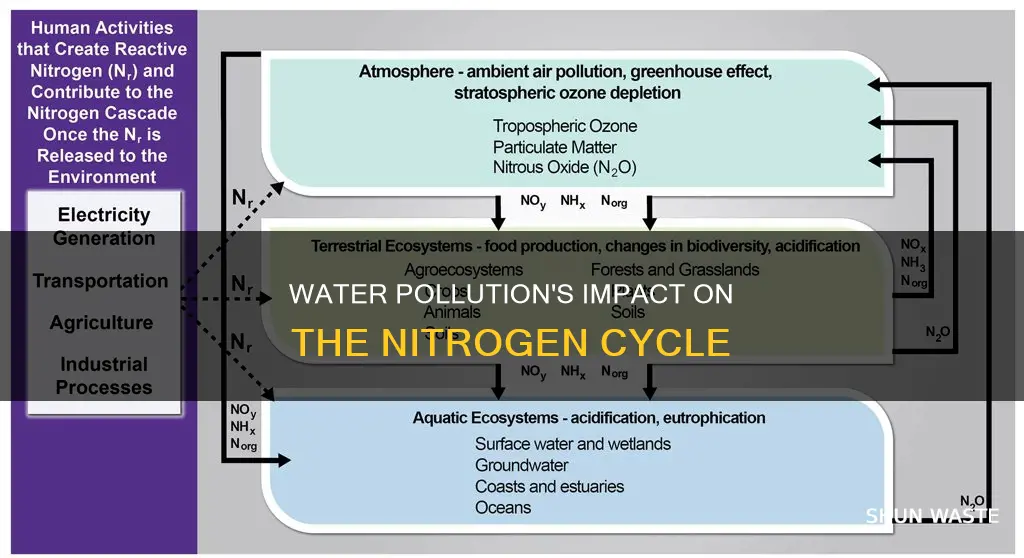
Nitrogen is the most abundant element in the Earth's atmosphere and is essential for life. However, human activities have significantly altered the global nitrogen cycle, leading to nitrogen pollution in water bodies. This occurs when excess nitrogen from sources such as fertilizers, air pollution, and manure enters aquatic ecosystems. Water pollution caused by nitrogen has far-reaching impacts on public health, the environment, and the economy. It can cause excessive growth of algae, known as algal blooms, which can deplete oxygen levels in the water, leading to fish kills and even dead zones where aquatic life cannot survive. Additionally, nitrogen pollution in drinking water sources can be harmful to human health, particularly for infants who are vulnerable to nitrate-induced conditions like blue baby syndrome. Understanding the effects of water pollution on the nitrogen cycle is crucial to mitigate these negative consequences and ensure the sustainability of aquatic ecosystems.
| Characteristics | Values |
|---|---|
| Excess nitrogen in water | Can cause eutrophication, leading to an increase in algae and aquatic plants, which can result in "dead zones" |
| Can cause a decrease in oxygen levels in water, leading to fish kills and a decline in aquatic life | |
| Can be harmful to human health, especially for infants who are vulnerable to nitrate compounds in drinking water | |
| Can impair our ability to breathe, limit visibility, and alter plant growth | |
| Can lead to nutrient imbalances and harm ecosystems and biodiversity | |
| Nitrogen pollution sources | Fertilizers, air pollution, manure, and industrial/agricultural activities |
| Rain can wash nitrates into watercourses and oceans | |
| Cars and power plants emit nitrogen oxides, which contribute to air pollution | |
| Impact on the nitrogen cycle | Human activities have significantly altered the global nitrogen cycle |
| Nitrogen fixation, nitrification, and denitrification are critical processes in the nitrogen cycle that can be affected by pollution | |
| Understanding the nitrogen cycle can help reduce pollution caused by excess fertilizer use |
What You'll Learn
- Excess nitrogen in water causes algal blooms, choking aquatic life
- Nitrogen-based pollution from agriculture and industry enters water systems
- Nitrogen pollution in groundwater can be harmful to human health
- Nitrogen contamination is more of a problem in shallow aquifers
- Excess nitrogen in soil that plants can't absorb can leach into groundwater

Excess nitrogen in water causes algal blooms, choking aquatic life
Nitrogen is the most abundant element in our atmosphere and is crucial to life. It is a key building block of DNA and is essential for plant growth. However, an excess of nitrogen can be harmful to plants and animals and can pollute aquatic systems.
One of the primary consequences of excess nitrogen in water is the stimulation of excessive algal growth, also known as algal blooms or harmful algal blooms (HABs). This occurs when there is an overabundance of nitrogen, a crucial nutrient for algae and aquatic plants, in waterways. Algal blooms can have detrimental effects on aquatic ecosystems, including reduced water quality, depletion of oxygen, and disruption of food resources and habitats.
The excessive growth of algae caused by high nitrogen levels can lead to the formation of thick, green layers on the water surface, often referred to as "scums" or "muck." These algal blooms can block sunlight from reaching underwater plants, negatively impacting their growth and disrupting the aquatic food chain. Additionally, as the algae decompose, they consume oxygen, leading to hypoxic or anoxic conditions in the water, which can be fatal for fish and other aquatic organisms.
The impact of excess nitrogen on algal blooms is particularly evident in the Gulf of Mexico, where nutrient pollution from the Mississippi River Basin has created a large "dead zone" that cannot support aquatic life due to extremely low oxygen levels. This dead zone, which occurs annually, is the largest in the United States, covering an area of about 6,500 square miles.
The problem of excess nitrogen in water is often attributed to human activities such as agriculture, where the overuse of fertilizers, animal manure, and soil erosion contribute to nutrient pollution. Additionally, wastewater treatment plants, stormwater runoff, and industrial facilities also play a role in releasing excess nitrogen into water bodies.
Shampoo's Water Pollution: What's the Real Damage?
You may want to see also

Nitrogen-based pollution from agriculture and industry enters water systems
Nitrogen is the most abundant element in the Earth's atmosphere and is crucial to life. It is a key component of DNA and supports the growth of plants, algae, and aquatic plants, which provide food and habitats for fish, shellfish, and smaller organisms. However, human activities have significantly altered the global nitrogen cycle, and nitrogen-based pollution from agriculture and industry is a major contributor to this issue.
Agricultural practices, such as the use of nitrogen fertilizers on crops, contribute to nitrogen-based pollution in water systems. While nitrogen fertilizers are essential for crop production, their excessive use can lead to pollution. It is estimated that 50% of nitrogen fertilizers added to farm fields end up as pollution, leaching into nearby water bodies. This process, known as leaching, occurs when forms of nitrogen, such as nitrate, dissolve in water and leak out of the soil. The application of ammonium-based fertilizers can also cause soil acidification, negatively impacting crop production and increasing the risk of nitrogen runoff into water bodies.
Industrial activities, including emissions from cars and power plants, also contribute to nitrogen-based pollution in water systems. These sources release nitrogen oxides (NOx) and ammonia, which are major air pollutants. When excess nitrogen returns to the earth from the atmosphere, it can contaminate forests, soils, and waterways. Additionally, industrial emissions have been linked to the increase in reactive nitrogen in the environment, impacting water bodies and biodiversity.
The impact of nitrogen-based pollution from agriculture and industry on water systems is significant. Excess nitrogen in water can cause adverse health and ecological effects. It can lead to eutrophication, resulting in unsightly scums of algae on the water surface and negatively impacting aquatic life. Algal blooms can deplete oxygen levels in the water, leading to fish kills and even "killing" lakes by depriving them of oxygen. Additionally, high levels of nitrate in drinking water sources can be harmful to human health, particularly for infants, who are at risk of developing methemoglobinemia, or "blue baby syndrome."
The understanding of the nitrogen cycle and the management of nitrogen-based pollution are crucial to mitigate these negative impacts. By recognizing the sources of pollution and implementing measures to reduce excessive nitrogen inputs into water systems, we can help protect aquatic ecosystems, maintain water quality, and safeguard human health.
How Tax Laws Can Help Reduce Water Pollution
You may want to see also

Nitrogen pollution in groundwater can be harmful to human health
Nitrogen is the most abundant element in the Earth's atmosphere and is crucial to life. It is a key building block of DNA and is essential for plant growth. However, an excess of nitrogen in the environment can have adverse effects on both ecological systems and human health. Nitrogen pollution in groundwater, in particular, can be harmful to human health in several ways.
Firstly, nitrogen pollution in groundwater can lead to the contamination of drinking water sources. High levels of nitrogen, especially in the form of nitrates, can be toxic to humans if consumed. This is especially true for infants, who are more vulnerable to the harmful effects of nitrates. Excessive nitrate consumption can also have negative health consequences for young livestock.
Secondly, nitrogen pollution in groundwater can contribute to the growth of algae and aquatic plants in water bodies. While these organisms are essential for providing food and habitats for aquatic life, their excessive growth can lead to a phenomenon known as algal blooms. Algal blooms can severely reduce or eliminate oxygen in the water, creating anaerobic conditions. This depletion of oxygen can lead to illnesses and even mass deaths among fish and other aquatic organisms, disrupting the delicate balance of aquatic ecosystems.
Moreover, the presence of algal blooms can also have direct adverse effects on human health. Elevated levels of toxins and bacterial growth associated with algal blooms can cause illnesses in humans if they come into contact with polluted water, consume contaminated fish or shellfish, or drink contaminated water. These toxins and bacteria can pose a significant risk to human health, potentially leading to various health issues.
Nitrogen pollution in groundwater is often a result of human activities, such as agricultural practices and fertilization. The excessive use of nitrogen-based fertilizers in agriculture can lead to nitrogen accumulation in soils and subsequent leaching into groundwater. This process, known as nitrogen leaching, contributes to the pollution of groundwater resources, which are crucial for drinking water supplies in many regions.
Protecting groundwater from nitrogen contamination is, therefore, a significant public health concern. It is essential to address the sources of nitrogen pollution, such as excessive fertilization, and implement sustainable practices to maintain the balance of this crucial element in our environment and safeguard human health.
Fast Fashion's Water Pollution: Understanding the Toxic Truth
You may want to see also

Nitrogen contamination is more of a problem in shallow aquifers
Nitrogen is a crucial element for all life on Earth, and it is the most abundant element in our atmosphere. It is a key building block of DNA and RNA, and is essential for plant growth. However, an overabundance of nitrogen in water can have adverse health and ecological effects. This is where the nitrogen cycle comes in: it helps us understand how nitrogen moves from the atmosphere to earth, through soils, and back into the atmosphere.
One of the primary ways that water pollution affects the nitrogen cycle is through the excessive use of nitrogen fertilizers. When too much fertilizer is added to soils, it can run off into nearby water bodies, causing an excess of nitrogen in these aquatic systems. This can lead to the overstimulation of aquatic plants and algae, which can clog water intakes, reduce oxygen levels, and block light from reaching deeper waters. This process is known as eutrophication and can result in the death of fish and other aquatic organisms, reducing biodiversity.
Additionally, water pollution from sources such as agricultural runoff and wastewater can introduce excess nitrogen into aquatic ecosystems. This can disrupt the natural balance of the nitrogen cycle, leading to an overabundance of nitrogen in the water. This excess nitrogen can then be converted into nitrate, which can contaminate groundwater, particularly in shallow aquifers.
Shallow aquifers are more susceptible to nitrogen contamination due to their proximity to the land surface. Contaminants can enter shallow aquifers directly through wells or openings in rocks, or indirectly through percolation, induced infiltration, or interaquifer leakage. Natural processes such as filtration, dispersion, and oxidation can help to degrade and remove contaminants, but they may not be effective for all types of pollutants.
To address the issue of nitrogen contamination in shallow aquifers, it is important to identify areas with a high risk of contamination and implement best management practices. This may include reducing the use of nitrogen fertilizers, using buffer zones of vegetation to filter nitrogen before it reaches water bodies, and improving wastewater treatment processes to remove excess nitrogen. By understanding the nitrogen cycle and taking proactive measures, we can help protect our aquatic ecosystems and ensure a sustainable supply of clean water.
Charged Particles: Unveiling Water Pollution Secrets
You may want to see also

Excess nitrogen in soil that plants can't absorb can leach into groundwater
Nitrogen is the most abundant element in our atmosphere and is crucial to life. It is a key element in nucleic acids DNA and RNA, which are essential to the growth of plants and animals. However, while nitrogen is essential, too much of it can be harmful. An excess of nitrogen in the soil can lead to water pollution, which in turn can negatively impact plant and animal life.
When there is too much nitrogen in the soil, plants may not be able to absorb it all. This excess nitrogen can then leach or drain into underground water sources, a process known as leaching. Leaching is where certain forms of nitrogen, such as nitrate, become dissolved in water and leak out of the soil, potentially polluting waterways. Sandy soils, for example, may lose nitrogen through leaching, while heavy, poorly drained soils may lose nitrogen through denitrification. Coarse-textured soils have a lower water-holding capacity and are therefore more susceptible to losing nitrate through leaching compared to fine-textured soils.
Excess nitrogen in the soil can come from a variety of sources, including fertilizer use, air pollution, and manure. When farmers add nitrogen fertilizer to their crops, they must be careful not to overapply it, as this can contaminate water sources with excess nitrate just before the crop enters a period of rapid growth. This excess nitrate can then be lost through leaching, potentially contributing to the pollution of water resources.
The pollution of water sources with excess nitrogen can have several adverse effects on the environment. It can cause eutrophication, leading to dense growths of aquatic plants and algae. These growths can clog water intakes, use up dissolved oxygen, and block light from reaching deeper waters. This can result in a decrease in animal and plant diversity and impact our ability to use the water for activities like fishing, swimming, and boating.
Additionally, an overabundance of nitrogen in water can be harmful to human health, particularly for young infants or livestock. Understanding the nitrogen cycle and managing nitrogen levels in the soil is crucial to protect our environment and ensure the health and well-being of both human and animal life.
Mining's Dark Side: Polluting Our Drinking Water Sources
You may want to see also
Frequently asked questions
Water pollution disrupts the natural nitrogen cycle by introducing excessive amounts of nitrogen into water bodies. This excess nitrogen, often from human activities, leads to eutrophication, causing algal blooms that deplete oxygen levels and create "dead zones" where aquatic life cannot survive.
Human activities such as agriculture, fertilizer use, and industrial processes are major sources of nitrogen pollution in water. Excessive use of nitrogen-based fertilizers on crops can lead to nitrogen runoff into nearby water bodies, contributing to the problem.
Nitrogen pollution in water has far-reaching ecological consequences. It disrupts the natural balance of aquatic ecosystems, leading to excessive growth of algae and aquatic plants. This, in turn, reduces oxygen levels, affecting the survival of fish and other aquatic organisms, and ultimately degrading the entire ecosystem.
To mitigate nitrogen pollution in water, it is essential to address the sources of pollution. This includes implementing better fertilizer management practices, reducing industrial emissions, and restoring natural habitats that can act as buffers, such as planting specific vegetation along riverbanks to absorb excess nitrogen before it reaches water bodies.











![Total Release Odor Fogger, Fresh N Clean - Black [66-306], 5 fl. oz.](https://m.media-amazon.com/images/I/61buFEAnzyL._AC_UL320_.jpg)







