The burning of coal for electricity generation is a significant contributor to environmental pollution. Coal combustion releases a multitude of harmful pollutants into the atmosphere, including sulfur dioxide, nitrogen oxides, and particulate matter, which can have detrimental effects on both human health and the environment. These emissions lead to air pollution, causing respiratory issues and contributing to the formation of smog and acid rain. Additionally, coal power plants are a major source of carbon dioxide emissions, a primary driver of climate change, as coal is a fossil fuel that releases greenhouse gases when burned. Understanding the pollution caused by coal-fired power generation is crucial in developing strategies to mitigate its impact and promote cleaner energy alternatives.
What You'll Learn
- Coal Combustion: Burning coal releases pollutants like sulfur dioxide and nitrogen oxides
- Ash Disposal: Coal ash contains toxic metals, posing risks to water and soil
- Acid Rain: Sulfur dioxide and nitrogen oxides cause acid rain, damaging ecosystems
- Greenhouse Gases: Coal combustion emits carbon dioxide, a major driver of climate change
- Particulate Matter: Coal-fired plants release fine particles, harmful to human health

Coal Combustion: Burning coal releases pollutants like sulfur dioxide and nitrogen oxides
The burning of coal for electricity generation is a significant contributor to air pollution, primarily due to the release of various harmful substances during the combustion process. When coal is burned, it undergoes a chemical reaction that produces a range of pollutants, including sulfur dioxide (SO2) and nitrogen oxides (NOx). These emissions are a major concern for environmental health and contribute to several adverse effects on both human and ecological systems.
Sulfur dioxide, a colorless gas with a strong odor, is released when sulfur present in coal reacts with oxygen during combustion. SO2 is a potent air pollutant and a precursor to the formation of acid rain. When released into the atmosphere, it can be transported over long distances, affecting regions far from the source of emission. Acid rain, caused by the reaction of SO2 with water vapor and other atmospheric components, can damage forests, aquatic ecosystems, and infrastructure.
Nitrogen oxides, including nitrogen dioxide (NO2), are also released during coal combustion. These gases are produced when nitrogen in the coal reacts with oxygen at high temperatures. NOx emissions contribute to the formation of ground-level ozone, a major component of smog, which has detrimental effects on human health and the environment. Exposure to ozone can cause respiratory issues, particularly in vulnerable populations such as children and the elderly. Moreover, nitrogen oxides play a crucial role in the creation of fine particulate matter (PM2.5), which can penetrate deep into the respiratory system and lead to various health problems.
The release of these pollutants during coal combustion has far-reaching consequences. Sulfur dioxide and nitrogen oxides contribute to the formation of fine particulate matter, which can be inhaled and cause respiratory and cardiovascular diseases. They also play a role in the degradation of air quality, leading to reduced visibility and the formation of haze, particularly in urban areas. The impact of these emissions extends beyond local environments, as they can contribute to regional and global air pollution, affecting climate patterns and ecosystems on a larger scale.
To mitigate the pollution caused by coal combustion, various strategies can be employed. These include implementing stricter emission standards and regulations, adopting cleaner combustion technologies, and transitioning to renewable energy sources. Capturing and controlling emissions through the use of flue-gas desulfurization and selective catalytic reduction systems can significantly reduce the release of sulfur dioxide and nitrogen oxides. Additionally, investing in energy efficiency measures and exploring alternative energy sources can help decrease the reliance on coal for electricity generation, ultimately leading to improved air quality and a healthier environment.
Urban Centers: The Hidden Sources of Environmental Degradation
You may want to see also

Ash Disposal: Coal ash contains toxic metals, posing risks to water and soil
The disposal of coal ash, a byproduct of coal-fired power plants, presents significant environmental challenges, particularly concerning its toxic metal content and its impact on water and soil quality. Coal ash, also known as fly ash, is a fine, powdery material that remains after coal is burned. It is a mixture of minerals and chemicals, including heavy metals such as lead, mercury, arsenic, and chromium. These toxic metals are of grave concern due to their potential to contaminate the environment and pose risks to human health.
When coal ash is disposed of in landfills or surface impoundments, it can leach these toxic metals into the surrounding soil and groundwater. Heavy metals like lead and mercury are particularly concerning as they can accumulate in the food chain, leading to long-term health issues for both wildlife and humans. For instance, mercury exposure can result in neurological disorders, while lead exposure can cause severe health problems, especially in children.
The leaching of these metals from coal ash is a significant environmental risk. As rain or irrigation water passes through the ash, it can dissolve and carry these toxic substances into nearby water bodies, including rivers, lakes, and groundwater. This process, known as leaching, can result in the contamination of drinking water sources and aquatic ecosystems. The presence of heavy metals in water can have detrimental effects on aquatic life, leading to population declines and potential ecological imbalances.
To mitigate these risks, proper ash disposal methods are essential. One approach is to use specialized containment systems that prevent leaching. These systems typically involve lining landfills or impoundments with impermeable materials to minimize the contact between the ash and water. Additionally, regular monitoring of groundwater and surface water quality is crucial to detect any contamination early and take corrective actions.
Furthermore, the recycling and safe disposal of coal ash can help reduce the environmental impact. Some of the metals in coal ash can be recovered and reused in various industries, while the remaining ash can be treated and stabilized to prevent leaching. Implementing these measures is vital to minimize the release of toxic metals into the environment and protect both water and soil resources from the harmful effects of coal ash disposal.
Beijing's Weather: A Double-Edged Sword for Air Quality
You may want to see also

Acid Rain: Sulfur dioxide and nitrogen oxides cause acid rain, damaging ecosystems
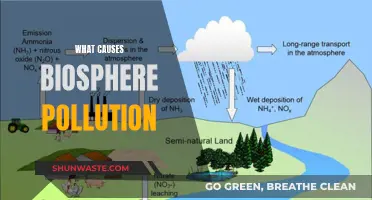
Acid rain is a significant environmental issue caused by the release of sulfur dioxide (SO2) and nitrogen oxides (NOx) into the atmosphere, primarily as a result of burning coal for electricity generation. These gases, when emitted, undergo chemical transformations, leading to the formation of acidic compounds that return to the Earth's surface in the form of rain, snow, fog, or even dry deposition. The process is a complex interplay of atmospheric chemistry and has far-reaching consequences for ecosystems, agriculture, and infrastructure.
The primary sources of sulfur dioxide and nitrogen oxides in the context of coal-fired power plants are the combustion of sulfur-containing minerals in coal and the various processes involved in electricity generation. When coal is burned, sulfur in the form of sulfur dioxide is released, contributing to acid rain. Similarly, nitrogen oxides are produced through the high-temperature combustion of coal and the subsequent cooling and expansion processes in the power plant's turbines. These gases are highly reactive and can undergo further chemical reactions in the atmosphere, forming more acidic compounds.
The formation of acid rain is a two-step process. Initially, SO2 and NOx react with water vapor and other atmospheric components to form sulfuric and nitric acids, respectively. These acids then condense onto tiny particles in the air, such as dust, pollen, and smoke, and are carried by wind to distant locations. When these acidic particles settle, they lower the pH of water bodies, making them more acidic. This change in water chemistry can have devastating effects on aquatic ecosystems, killing fish and other aquatic organisms and disrupting the entire food chain.
The impact of acid rain on ecosystems is profound and wide-ranging. It can lead to the decline or even disappearance of sensitive plant and animal species, particularly in freshwater ecosystems like lakes and streams. Acid rain also contributes to the degradation of forests, making trees more susceptible to disease, insect infestations, and extreme weather events. Moreover, it can damage agricultural crops, reducing yields and affecting food production. The economic implications are significant, as the restoration and maintenance of affected ecosystems can be costly, and the loss of agricultural productivity can have social and economic repercussions.
Addressing the issue of acid rain requires a multifaceted approach. One key strategy is the reduction of sulfur dioxide and nitrogen oxide emissions from coal-fired power plants. This can be achieved through the implementation of advanced emission control technologies, such as flue-gas desulfurization for SO2 and selective catalytic reduction for NOx. Additionally, transitioning to cleaner energy sources, such as renewable energy, can significantly decrease the reliance on coal and, consequently, the emissions contributing to acid rain. Public awareness and policy interventions also play a crucial role in promoting sustainable practices and mitigating the environmental impacts of coal-based electricity generation.
Animal Farming's Impact: A Hidden Environmental Cost
You may want to see also

Greenhouse Gases: Coal combustion emits carbon dioxide, a major driver of climate change
The burning of coal for electricity generation is a significant contributor to greenhouse gas emissions, primarily carbon dioxide (CO2). This process is a major driver of climate change, as the release of CO2 intensifies the natural greenhouse effect, leading to global warming and associated environmental impacts. When coal is burned, it undergoes a complex chemical reaction, releasing various pollutants, including CO2, into the atmosphere. This emission of CO2 is a direct result of the combustion process, where coal's carbon content is oxidized, producing energy and heat.
The combustion of coal is an inefficient process, as it releases a significant amount of CO2 for every unit of electricity generated. On average, coal-fired power plants emit around 2.1 pounds of CO2 for each kilowatt-hour of electricity produced. This is much higher than other energy sources, such as natural gas and renewable alternatives, which have lower carbon footprints. The excessive CO2 emissions from coal combustion are a critical concern as they contribute to the accumulation of greenhouse gases in the Earth's atmosphere.
Greenhouse gases, including CO2, have a unique property that makes them effective at trapping heat. As CO2 is released into the atmosphere, it acts like a blanket, allowing sunlight to pass through but trapping some of the heat that would otherwise escape back into space. This natural greenhouse effect is essential for maintaining the planet's temperature and supporting life as we know it. However, human activities, particularly the burning of fossil fuels like coal, have significantly increased the concentration of greenhouse gases, leading to an enhanced greenhouse effect and global warming.
The impact of increased CO2 levels is far-reaching. It leads to rising global temperatures, melting ice caps, and sea-level rise, among other consequences. These changes disrupt ecosystems, impact agriculture, and pose risks to human health and infrastructure. To mitigate these effects, it is crucial to reduce CO2 emissions, especially from coal-fired power plants. Transitioning to cleaner and more sustainable energy sources, such as wind, solar, and hydroelectric power, can significantly decrease the reliance on coal and other fossil fuels, thereby reducing the emission of greenhouse gases and combating climate change.
In summary, coal combustion for electricity generation is a major source of CO2 emissions, a potent greenhouse gas. This process contributes to climate change by intensifying the greenhouse effect, leading to global warming and its associated environmental challenges. Addressing this issue requires a shift towards cleaner energy sources and improved energy efficiency to reduce the carbon footprint of the electricity sector.
Roadkill's Impact: Uncovering the Hidden Environmental Cost
You may want to see also

Particulate Matter: Coal-fired plants release fine particles, harmful to human health
Coal-fired power plants are a significant source of particulate matter (PM) pollution, which poses a serious threat to human health and the environment. When coal is burned, it releases a variety of fine particles, including soot, ash, and other microscopic fragments. These particles are classified as PM2.5, indicating their extremely small size, typically 2.5 micrometers or less in diameter. This small size allows them to penetrate deep into the respiratory system, causing severe health issues.
The impact of these fine particles on human health is profound. When inhaled, PM2.5 can reach the alveoli, the tiny air sacs in the lungs where gas exchange occurs. This can lead to a range of respiratory problems, including asthma, bronchitis, and reduced lung function. Prolonged exposure to high levels of PM2.5 has been linked to increased risks of heart disease, lung cancer, and even premature death. Vulnerable populations, such as children, the elderly, and individuals with pre-existing health conditions, are particularly at risk.
The sources of particulate matter from coal-fired plants are diverse. During the combustion process, coal releases sulfur dioxide (SO2) and nitrogen oxides (NOx), which can react with other substances in the atmosphere to form secondary particles. These secondary particles, including sulfate aerosols and nitrate aerosols, contribute significantly to the overall PM2.5 load. Additionally, the physical combustion process itself generates solid particles, such as fly ash and bottom ash, which are released directly into the air.
The environmental consequences of particulate matter from coal-fired plants are far-reaching. PM2.5 can contribute to the formation of smog, reducing visibility and creating a haze that obscures natural landscapes. It also plays a role in the acidification of water bodies, as the particles can carry and release acidic compounds when they settle on water surfaces. Furthermore, the deposition of PM2.5 on vegetation and soil can have detrimental effects on ecosystems, affecting plant growth and the overall health of natural habitats.
Addressing the issue of particulate matter from coal-fired plants requires a multi-faceted approach. Implementing stricter emission standards and regulations is essential to limit the release of harmful particles. This includes adopting advanced technologies for pollution control, such as electrostatic precipitators and fabric filters, which can capture fine particles before they are released into the atmosphere. Additionally, transitioning to cleaner energy sources, such as renewable energy and natural gas, can significantly reduce the reliance on coal and, consequently, the emission of particulate matter.
Unraveling Kevlar's Environmental Impact: A Pollution Perspective
You may want to see also
Frequently asked questions
Coal combustion releases a range of pollutants into the atmosphere, including sulfur dioxide (SO2), nitrogen oxides (NOx), and particulate matter (PM). These emissions are a major source of air pollution, leading to respiratory issues and contributing to the formation of smog and acid rain.
Coal-fired power plants require substantial amounts of water for cooling, which can lead to the discharge of heated wastewater into nearby water bodies. This process can disrupt aquatic ecosystems, harm fish and other aquatic organisms, and contribute to the contamination of drinking water sources.
Yes, coal mining and processing can result in the release of toxic substances, such as heavy metals (e.g., lead, mercury) and acidic drainage, which can pollute nearby rivers, streams, and groundwater. These pollutants can have severe ecological and health consequences.
Coal-fired power plants are a significant source of greenhouse gas emissions, primarily carbon dioxide (CO2). The burning of coal releases large amounts of CO2, a potent greenhouse gas, which traps heat in the atmosphere, leading to global warming and climate change.
Absolutely. Coal mining can result in habitat destruction, land degradation, and the displacement of wildlife. Additionally, coal transportation and storage can lead to soil and water contamination. The entire process, from mining to combustion, has significant environmental and ecological footprints.