Motor vehicles are a major source of air pollution, with a study from Harvard University attributing nearly 20,000 deaths in 2017 to their emissions. As a result, there is a growing interest in alternative fuels to gasoline, such as methanol, which is made from natural gas, coal, or biomass. Methanol has been found to be a more efficient fuel than gasoline, with a higher octane rating, and it is safer for the environment as it emits less air pollution and does not contain carcinogenic BTEX. However, it provides fewer miles to the gallon and has a lower energy density. While methanol is a promising alternative fuel, it also has its drawbacks, such as increased engine corrosion and higher fuel consumption.
| Characteristics | Values |
|---|---|
| Safety | Methanol is less likely to ignite and is safer for the environment than gasoline. It is also less likely to result in a deadly car fire. |
| Health | Gasoline is attributed to more than 50% of cancers caused by outdoor air pollution sources. Methanol emits less air pollution. |
| Greenhouse gases | Methanol produces less nitrogen oxide, a greenhouse gas, than gasoline. However, if methanol is made from natural gas, it will produce a similar amount of carbon dioxide per unit of energy as gasoline. |
| Energy | Methanol has a higher octane rating than gasoline, resulting in higher thermal efficiency and power output. However, it has lower energy density, resulting in fewer miles to the gallon. |
| Cost | Methanol is less expensive to sustainably produce than gasoline. |
| Fire visibility | Methanol fires are often invisible in daylight, but additives can be used to increase visibility. |
| Corrosiveness | Methanol is more corrosive than gasoline. |
| Volatility | Methanol is less volatile than gasoline and burns at a lower temperature. |
What You'll Learn

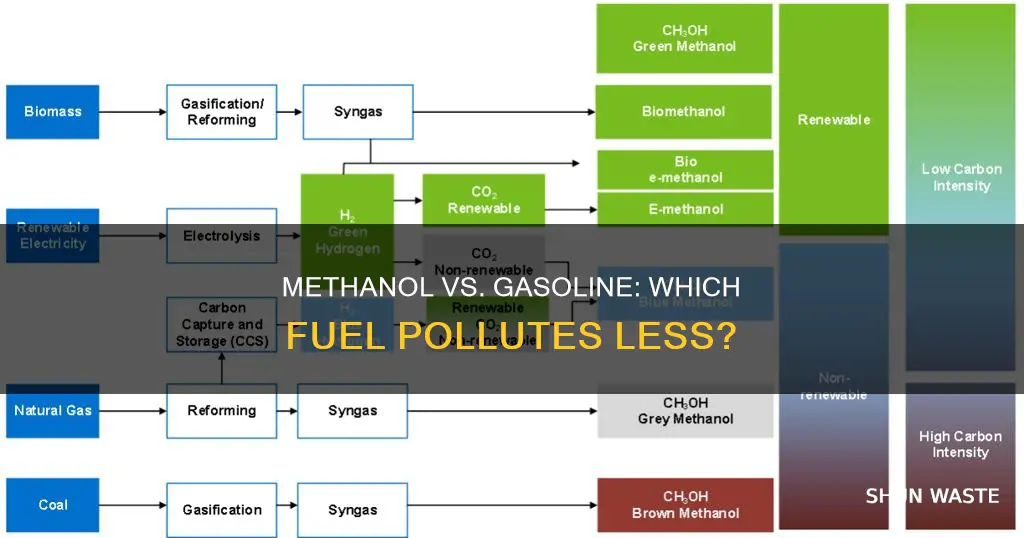
Methanol is made from natural gas, coal, or biomass
Methanol is a fuel that can be made from natural gas, coal, or biomass. It is produced by companies such as Methanex and Celanese, with the former producing the majority of its methanol from natural gas. Natural gas is a non-renewable fossil fuel feedstock, although it is possible to produce renewable natural gas from sources such as landfills, sewage plants, and animal manure farms.
Methanol can also be made using carbon capture technology, with renewable feedstocks, or from green hydrogen combined with recycled carbon dioxide (CO₂). The use of carbon capture technology can result in a product with significantly lower emissions than conventionally produced methanol, known as blue methanol.
When made from natural gas, methanol produces a similar amount of carbon dioxide per unit of energy used as gasoline. On the other hand, when methanol is made from coal, it can result in up to twice the amount of carbon dioxide emissions compared to gasoline.
While methanol-gasoline mixtures can reduce emissions of certain pollutants, such as carbon monoxide, the overall impact on pollution depends on various factors, including the feedstock used to produce the methanol and the efficiency of the vehicles utilizing it.
Pollution's Impact: A Global Concern?
You may want to see also

It reduces emissions of some air pollutants, especially carbon monoxide
Motor vehicles are a major source of air pollution, producing over one-third of the carbon monoxide and nitrogen oxide gases in the atmosphere. This air pollution carries significant risks to human health and the environment. A study from Harvard University attributed nearly 20,000 deaths in 2017 to motor vehicle emissions.
Methanol is a more efficient fuel than gasoline and can be used as an alternative biofuel for internal combustion engines, either in combination with gasoline or independently. It is made from natural gas, coal, or biomass. When used in combination with gasoline, methanol reduces emissions of some air pollutants, particularly carbon monoxide.
Methanol has a higher octane rating than gasoline, which allows it to achieve a higher thermal efficiency and power output. However, it also has a lower energy density, which results in higher fuel consumption. Methanol is also safer than gasoline as it has a higher ignition temperature and is less likely to result in a deadly car fire.
While methanol is a more efficient and safer fuel than gasoline, it is not a perfect solution to the problem of air pollution. For example, methanol burning will increase emissions of carbon dioxide, a major cause of global warming and climate change. If methanol is made from coal, it will double carbon dioxide emissions compared to gasoline.
Do Trains Pollute Less Than Trucks?
You may want to see also

It is safer than gasoline, with a higher ignition point
The search results show that methanol is generally considered a safer and less polluting alternative to gasoline. Firstly, methanol has a higher ignition point than gasoline, making it harder to ignite and therefore less likely to result in a deadly car fire. Methanol vapors will only ignite at an airborne concentration four times greater than that of gasoline vapors.
Secondly, methanol produces one-eighth the heat of gasoline, and its combustion generally produces fewer greenhouse gases in the form of nitrogen oxides. Additionally, methanol blends in gasoline have been shown to reduce emissions of certain air pollutants, particularly carbon monoxide and unburnt hydrocarbons.
However, it is important to note that if methanol is made from natural gas, it will produce a similar amount of carbon dioxide per unit of energy used as gasoline. If produced from coal, methanol will double carbon dioxide emissions.
While methanol is considered safer and less polluting than gasoline, it is not a perfect fuel. It has a lower energy density, providing fewer miles per gallon, and it can be corrosive to older automobile tubing and casing. Nevertheless, methanol is a renewable and efficient alternative to gasoline, and its use can lead to improved air quality and reduced health risks associated with motor vehicle emissions.
Steam Engines and Pollution: Factorio's Environmental Impact
You may want to see also

It is more efficient than gasoline, but provides less fuel economy
Methanol is a more efficient fuel than gasoline, but it provides less fuel economy. This is due to its lower energy density, which results in higher fuel consumption. While methanol stores half the energy of traditional gasoline, it burns more cleanly, with no soot, particulates, or other residues. It also has a higher octane rating, allowing it to achieve greater thermal efficiency and power output in engines designed for methanol use.
Methanol is made from natural gas, coal, or biomass. It can be used as an alternative biofuel for internal combustion engines, either in combination with gasoline or on its own. Methanol has been of great interest as a potential replacement for gasoline due to its environmental benefits. It emits less air pollution, particularly carbon monoxide, and does not contain the carcinogenic BTEX found in gasoline.
However, it is important to note that the production of methanol from natural gas results in similar carbon dioxide emissions per unit of energy used as gasoline. If produced from coal, methanol production can double carbon dioxide emissions. Additionally, methanol's lower energy density means that it provides fewer miles per gallon, impacting fuel economy.
Despite these considerations, methanol has several advantages over gasoline. It is safer, with a higher ignition temperature and a lower likelihood of ignition. It also acts as an anti-freeze agent, prevents dirt and grime buildup within the engine, and is effective in fighting methanol-based fires.
Overall, while methanol is more efficient than gasoline in terms of reducing certain emissions and providing higher thermal efficiency, it does come with the trade-off of lower fuel economy due to its lower energy density.
Solar Panels: Earth-Friendly Energy or Polluting Power?
You may want to see also

It is less likely to ignite and cause a deadly car fire
Methanol is a less polluting fuel than gasoline. It is also safer, as it is far more difficult to ignite than gasoline and burns about 60% slower. A methanol fire releases energy at around 20% of the rate of a gasoline fire, resulting in a much cooler flame. This makes it a much less dangerous fire that is easier to contain with proper protocols.
Unlike gasoline fires, water is acceptable and even preferred as a fire suppressant for methanol fires. This is because water cools the fire and rapidly dilutes the fuel below the concentration where it will maintain self-flammability. These facts mean that, as a vehicle fuel, methanol has great safety advantages over gasoline.
Methanol is also safer in the event of an accident, as it does not produce an opaque cloud of smoke, making it the primary fuel source for IndyCar, Monster Trucks, and USAC sprint cars. It is also used as the primary fuel ingredient in the powerplants of radio-controlled model aircraft, cars, and trucks.
However, it is important to note that methanol is more toxic than gasoline and has a lower energy density, resulting in fewer miles per gallon. It is also less volatile and burns at a lower temperature, making it more difficult to start and warm up an engine in cold weather. Methanol may also contain soluble contaminants like chloride ions, which can make it more corrosive.
While methanol is generally less polluting than gasoline, it is not a perfect solution. For example, methanol burning can increase emissions of carbon dioxide, a major cause of greenhouse warming and global climate change, especially if it is made from coal. Additionally, it may not significantly improve air quality in cities, as it does not address non-vehicular sources of air pollution.
Air Quality Index Calculation in the USA Explained
You may want to see also
Frequently asked questions
Yes, methanol is less polluting than gasoline. It emits less air pollution and does not contain the carcinogenic BTEX found in gasoline.
Methanol is made from natural gas, coal, or biomass. It is a more efficient fuel than gasoline and produces clearer smoke. It also has a higher octane rating, which can achieve a higher thermal efficiency and power output. However, it provides fewer miles to the gallon due to its lower energy density.
Methanol can help reduce emissions of some air pollutants, particularly carbon monoxide, which is a major source of air pollution in cities. However, it is important to note that methanol burning will increase emissions of carbon dioxide, a major cause of global warming and climate change.
Methanol fuel has some safety advantages over gasoline. For example, it is less likely to ignite and is less volatile, making it safer to handle. However, it requires safe handling and can be absorbed through the skin, so appropriate personal protective equipment (PPE) is necessary.