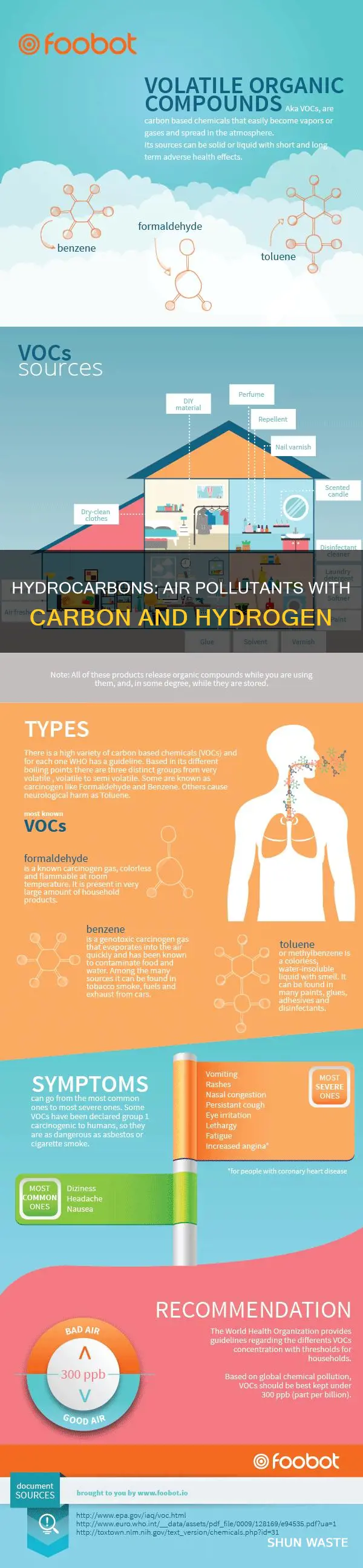
Air pollution is a pressing issue that has detrimental effects on human health, the environment, and property. It involves the release of various substances, including gases, finely divided solids, and liquid aerosols, into the atmosphere at rates that surpass the environment's capacity to dilute or absorb them. Among the numerous air pollutants, one that contains both carbon and hydrogen is methane (CH4). Methane is a trace gas that is naturally occurring but can also be produced by human activities, such as the combustion of fossil fuels and agricultural practices. It contributes to the greenhouse effect and plays a significant role in global warming.
| Characteristics | Values |
|---|---|
| Name of Air Pollutant | Carbon monoxide (CO) |
| Composition | Inhalable particles composed of sulphate, nitrates, ammonia, sodium chloride, black carbon, mineral dust, or water |
| Health Impact | CO is a colourless, odourless gas that can aggravate respiratory diseases. It is produced by the incomplete combustion of carbonaceous fuels such as wood, petrol, etc. |
| Other Information | CO is a major component of PM2.5 and is sometimes referred to as soot. It is emitted from anthropogenic sources such as diesel vehicles and biomass cookstoves. |
What You'll Learn
- Black carbon, or soot, is a major component of PM2.5
- Carbon monoxide is a colourless, odourless gas produced by the incomplete combustion of carbonaceous fuels
- Nitrogen oxides are emitted when hydrogen is combusted at high temperatures
- Nitrogen dioxide is emitted by vehicles and reacts with oxygen to form tropospheric ozone
- Sulfur dioxide is emitted by the burning of fossil fuels and combines with water vapour to form acid rain

Black carbon, or soot, is a major component of PM2.5
Air is a mixture of naturally occurring gases and human-made pollutants. The troposphere, the lowest layer of the Earth's atmosphere, contains three-fourths of all air. While the dry composition of the atmosphere is mostly nitrogen and oxygen, it also contains fractional amounts of other gases, including carbon dioxide, methane, and hydrogen.
One of the major air pollutants is particulate matter (PM), which refers to inhalable particles composed of sulphate, nitrates, ammonia, sodium chloride, black carbon, mineral dust, or water. These particles can be of various sizes, with PM2.5 and PM10 being the most common and relevant for health. PM2.5 particles, which are 2.5 microns or less in diameter, can penetrate deep into the lungs and enter the bloodstream, causing serious health issues such as cardiovascular and respiratory problems.
Black carbon, or soot, is a significant component of PM2.5. It is produced by the incomplete combustion of fossil fuels, biofuels, and biomass. Both anthropogenic and natural sources contribute to black carbon emissions. Diesel vehicles, biomass cookstoves, and wildfires are examples of these sources. Black carbon is a potent warming agent, contributing to environmental disruption and accelerating glacier melting.
The health risks associated with black carbon are significant. Short- and long-term exposure has been linked to adverse cardiovascular effects and increased mortality rates. Black carbon particles can settle on plant leaves, increasing their surface temperature, reducing sunlight reaching the Earth, and interfering with rainfall patterns. This interference can have far-reaching consequences for ecosystems and human livelihoods, particularly in regions that rely on monsoons for agriculture.
Additionally, black carbon, when combined with tropospheric ozone, contributes to substantial staple crop losses globally. The toxicity of PM2.5 is enhanced by the presence of black carbon aggregates, which have a high adhesion ability to absorb other particles, including toxic heavy metals. This complexity further exacerbates the health impacts of PM2.5 exposure.
Energy Conservation: Air Pollution's Ally or Adversary?
You may want to see also

Carbon monoxide is a colourless, odourless gas produced by the incomplete combustion of carbonaceous fuels
Carbon monoxide (CO) is a harmful and colourless gas that is also odourless. It is produced by the incomplete combustion of carbon-containing fuels, such as wood, petrol, and other fossil fuels. This makes carbon monoxide a common air pollutant, particularly in urban areas, where it is emitted directly into the air from burning fuel oil, gasoline, and natural gas in power plants, automobiles, and other combustion sources.
The danger of carbon monoxide lies in its invisible and undetectable nature by humans. It is hazardous to human health, and exposure to it can have serious consequences. Carbon monoxide is a significant component of indoor and outdoor air pollution, often produced by household activities such as cooking and heating with dirty technologies, as well as high-temperature combustion in vehicles, industries, and power-generating facilities.
Carbon monoxide's presence in the air can lead to adverse health effects, including respiratory issues and other long-term problems. It is a key contributor to the formation of ground-level ozone (O3), which is a harmful air pollutant and a primary ingredient in smog. Ground-level ozone is formed through photochemical reactions between pollutants such as volatile organic compounds, carbon monoxide, and nitrogen oxides emitted from vehicles and industrial sources.
The combustion of carbonaceous fuels, such as wood and petrol, releases carbon monoxide into the atmosphere. This incomplete combustion occurs when there is insufficient oxygen to fully react with the carbon present in the fuel, resulting in the formation of carbon monoxide instead of carbon dioxide. This process is common in household activities, industrial processes, and vehicle engines, contributing to ambient air pollution.
Additionally, carbon monoxide plays a significant role in the formation of secondary pollutants. It reacts with other chemicals and atmospheric compounds, leading to the creation of new harmful substances. These reactions can further degrade air quality and pose additional risks to human health and the environment. Overall, carbon monoxide is a significant contributor to air pollution and has detrimental effects on the atmosphere and human well-being.
Air Quality Alert: Polluted Air in New York City
You may want to see also

Nitrogen oxides are emitted when hydrogen is combusted at high temperatures
Nitrogen oxides (NOx) are formed when nitrogen and oxygen react during the combustion of fuels in the presence of air at high temperatures. This process occurs in car engines and power stations, for example. NOx is a hazardous air pollutant and is a significant source of air pollution, particularly in large cities with high motor vehicle traffic.
Hydrogen combustion can produce NOx, including nitric oxide (NO) and nitrogen dioxide (NO2). As hydrogen burns at a higher temperature than natural gas, its combustion may result in higher NOx emissions. However, hydrogen has a larger stable combustion temperature range, allowing for a higher ratio of air to fuel, which dilutes the hydrogen and lowers the combustion temperature, thereby reducing NOx emissions.
NOx formation is highly temperature-dependent. At high temperatures, nitrogen and oxygen undergo an endothermic reaction to produce various oxides of nitrogen. This reaction occurs during the combustion of a mixture of air and fuel, such as in an internal combustion engine or a power station boiler.
While hydrogen combustion can produce NOx, there are strategies to prevent or reduce these emissions. For example, flue gas treatment can convert harmful emissions into less harmful compounds, similar to the process used in catalytic converters for gasoline and diesel vehicles. Additionally, the larger stable combustion temperature range of hydrogen can help reduce NOx emissions by allowing for more effective dilution of the fuel.
Nitrogen oxides have several negative impacts on the environment and human health. They contribute to the formation of smog, acid rain, and tropospheric ozone depletion, which is hazardous to plant and animal cells. Exposure to nitrogen dioxide (NO2), an important precursor of ozone, can irritate airways and aggravate respiratory diseases.
Mexico's Air Pollution: Strategies for a Cleaner Future
You may want to see also

Nitrogen dioxide is emitted by vehicles and reacts with oxygen to form tropospheric ozone
Air is a mixture of naturally occurring gases and human-made air pollutants. The troposphere, the lowest layer of the Earth's atmosphere, contains about 78% nitrogen, 21% oxygen, and trace amounts of other gases, including methane, carbon dioxide, and hydrogen. Human activities, such as burning fossil fuels and vehicle emissions, introduce pollutants into the air, degrading its quality and causing health issues.
One such pollutant is nitrogen dioxide (NO2), which is commonly emitted by vehicles. High-temperature combustion in automobiles, industries, and power-generating facilities contributes to ambient air pollution. Nitrogen dioxide is a significant component of vehicle exhaust, along with other harmful chemicals like carbon monoxide and sulfur dioxide.
Nitrogen dioxide, upon its release into the atmosphere, reacts with oxygen. This chemical reaction results in the formation of tropospheric ozone (O3). Ozone is a highly reactive gas composed of three oxygen atoms. While stratospheric ozone in the upper atmosphere protects us from harmful ultraviolet rays, tropospheric ozone at ground level is a harmful air pollutant.
Tropospheric ozone is a key component of smog, which is harmful to both human and plant life. It can cause respiratory issues, trigger asthma, reduce lung function, and lead to lung disease. The presence of ground-level ozone is not due to direct emissions but is a byproduct of chemical reactions between oxides of nitrogen (NOx) and volatile organic compounds (VOCs). These reactions occur in the presence of sunlight, leading to higher ozone levels during sunny weather.
Ozone pollution is not limited to urban areas but can also affect rural regions due to wind transportation. The EPA works to improve air quality by designating areas as attainment or nonattainment based on national ambient air quality standards. States with nonattainment areas must develop plans to reduce emissions and improve ozone levels. These collective efforts are crucial in mitigating the health and environmental impacts of tropospheric ozone formation from nitrogen dioxide emissions.
Breathe Easy: Survival Guide for Delhi's Air Pollution
You may want to see also

Sulfur dioxide is emitted by the burning of fossil fuels and combines with water vapour to form acid rain
Sulfur dioxide (SO2) is a major air pollutant that is emitted during the burning of fossil fuels containing sulfur. It is a colorless gas with a pungent smell, often associated with the odor of burnt matches. The combustion of sulfur-bearing fossil fuels, such as fuel oil, gasoline, and natural gas, in power plants, automobiles, and other industrial facilities, is the primary source of SO2 emissions.
SO2 is not only a direct byproduct of burning sulfur-containing fuels but also arises from specific industrial processes. These include metal refining, metal extraction from ore, and the burning of sulfur itself. Additionally, natural sources such as volcanic activity contribute to SO2 emissions.
Once released into the atmosphere, SO2 combines with other compounds and reacts with water vapour, leading to the formation of secondary pollutants. These include sulfate aerosols, particulate matter, and acid rain. Acid rain is a significant environmental concern, causing damage to both natural and human-made environments, including trees, plants, and culturally important objects like statues and monuments.
The presence of SO2 in the atmosphere has implications for human health as well. Short- and long-term exposure to SO2 can contribute to respiratory illness and exacerbate existing heart and lung conditions. Recognizing the health and environmental risks associated with SO2, regulatory bodies have implemented measures to reduce SO2 emissions. These include removing sulfur from fuels before combustion, employing fuel additives, and implementing federal regulations to lower the sulfur content in fuels.
In summary, sulfur dioxide (SO2) is a harmful air pollutant produced by the burning of fossil fuels and other sulfur-containing materials. Its release into the atmosphere leads to the formation of acid rain and other secondary pollutants, causing environmental degradation and posing risks to human health. Efforts to mitigate SO2 emissions are ongoing, aiming to minimize its impact on the environment and human well-being.
Air Pollution's Environmental Racism in the Bay Area
You may want to see also
Frequently asked questions
Carbon monoxide (CO) is a colourless, odourless gas that is produced by the incomplete combustion of carbonaceous fuels such as wood and petrol. It is a common air pollutant that is harmful to human health.
Exposure to carbon monoxide can be extremely dangerous as it is a toxic gas. It can cause serious adverse health effects, including chest pain, coughing, throat irritation, and airway inflammation. It is also linked to an increased risk of respiratory conditions such as asthma and lung disease.
Carbon monoxide is released into the atmosphere through the combustion of fossil fuels, such as fuel oil, gasoline, and natural gas. This includes activities such as vehicle exhaust emissions, industrial processes, and power generation.







