Ozone air pollution, also known as ground-level ozone, is a harmful air pollutant and the main ingredient in smog. Ground-level ozone is not emitted directly into the air but is formed through chemical reactions between oxides of nitrogen (NOx) and volatile organic compounds (VOCs) in the presence of sunlight. These gaseous compounds mix in the ambient air, and when they interact with sunlight, ozone is formed. Ozone can have serious health effects on humans, especially those with asthma, and it can also harm vegetation and ecosystems.
| Characteristics | Values |
|---|---|
| What is ozone air pollution | Ozone is one of the six common air pollutants identified in the Clean Air Act. |
| What is ground-level ozone | Ground-level ozone is formed just above the earth's surface (up to about 2 miles above ground) and impacts human, animal, and plant respiration. |
| What is stratospheric ozone | Stratospheric ozone forms 6-30 miles above the earth's surface when intense sunlight causes oxygen molecules (O2) to break up and reform as ozone molecules (O3). |
| How is ground-level ozone formed | Ground-level ozone is formed by chemical reactions between oxides of nitrogen (NOx) and volatile organic compounds (VOCs) in the presence of sunlight. |
| What are the sources of ground-level ozone | Ground-level ozone comes from pollution emitted by cars, power plants, industrial boilers, refineries, chemical plants, paints, cleaners, solvents, and motorized lawn equipment. |
| What are the health effects of ozone pollution | Ozone can damage the tissues of the respiratory tract, causing inflammation and irritation, and result in symptoms such as coughing, chest tightness, and worsening of asthma symptoms. |
| Who is most at risk from ozone pollution | People at greatest risk of harm from breathing air containing ozone include people with asthma, children, older individuals, and adults with respiratory problems. |
| How to protect oneself from ozone pollution | Individuals can take steps to protect themselves on days with unhealthy levels of air pollutants and ask policymakers to require cleanup of air pollution. |
What You'll Learn
- Ground-level ozone is caused by chemical reactions between nitrogen oxides and volatile organic compounds in sunlight
- Sources of nitrogen oxides include cars, power plants, industrial boilers, refineries, and chemical plants
- Ozone pollution is especially harmful to people with asthma, metabolic disorders, and lung diseases
- Ozone is one of the six common air pollutants identified in the Clean Air Act
- Ozone is an invisible, odourless gas, and the most widespread pollutant in the US

Ground-level ozone is caused by chemical reactions between nitrogen oxides and volatile organic compounds in sunlight
Ozone is a colourless gas made up of three oxygen atoms. Ground-level ozone is a harmful air pollutant and the main ingredient in "smog". It is not emitted directly into the air but is instead created by chemical reactions between nitrogen oxides and volatile organic compounds in the presence of sunlight.
Nitrogen oxides (NOx) are a group of highly reactive gases, including nitrogen dioxide, nitrous acid, and nitric acid. Nitrogen dioxide is commonly produced by fuel combustion, such as in vehicles, power plants, and off-road equipment. These sources of nitrogen oxides combine with volatile organic compounds (VOCs) to form ground-level ozone. VOCs are emitted from a variety of sources, including industrial boilers, refineries, and chemical plants, as well as paints, cleaners, solvents, and even lawn equipment.
When these gaseous compounds mix in the ambient air and interact with sunlight, ozone is formed. Hot, sunny, and calm weather further promotes ozone formation. Ground-level ozone forms just above the Earth's surface, up to about two miles high, and negatively impacts human, animal, and plant respiration. It can cause inflammation and irritation of the respiratory tract, resulting in symptoms like coughing, chest tightness, and worsening of asthma symptoms.
The US EPA has designated standards for ground-level ozone concentrations, aiming to protect both human health and public welfare, including vegetation. Areas that meet or exceed these standards are called attainment areas, while those that do not meet them are nonattainment areas. Efforts to reduce emissions of pollutants that form ground-level ozone include vehicle and transportation standards, regional haze and visibility rules, and regular reviews of the standards.
Human Activities Polluting the Air and Our Health
You may want to see also

Sources of nitrogen oxides include cars, power plants, industrial boilers, refineries, and chemical plants
Nitrogen oxides (NOx) are a group of highly reactive gases, including nitrogen dioxide, nitrous acid, and nitric acid. They are produced from fuel combustion in both mobile and stationary sources. Cars, trucks, and buses are the largest sources of NOx emissions, with diesel-powered non-road equipment and industrial processes such as oil and gas production following closely behind. In large cities, nitrogen oxides are also produced from stationary emissions, such as coal-fired power plants and electric power plant boilers.
Industrial boilers, which are used in many industries, are another significant source of nitrogen oxide emissions. These boilers burn fossil fuels like coal, oil, and methane gas (natural gas) at high temperatures, releasing nitrogen dioxide and other nitrogen oxides into the atmosphere. Refineries and chemical plants also contribute to nitrogen oxide emissions through the combustion of fuels and other industrial processes.
Power plants are a major source of nitrogen oxide emissions, particularly those that burn fossil fuels like coal and oil. The combustion of these fuels releases nitrogen oxides into the atmosphere, contributing to air pollution. In addition to traditional power plants, gas-fired power plants, and facilities that extract, process, or transport oil and gas can also emit significant amounts of nitrogen oxides if they burn fuels in flares.
Nitrogen oxides are also produced during the extraction, processing, and transportation of oil and gas. This includes both indoor and outdoor sources, such as unvented heaters, gas stoves, and flares used during extraction and processing. High outdoor NOx levels originating from local traffic and other combustion sources can also influence indoor levels.
Nitrogen oxides are a significant contributor to air pollution and have harmful effects on human health. They are a primary pollutant and a component of secondary pollutants, such as ozone, which is formed through chemical reactions between nitrogen oxides and volatile organic compounds (VOCs) in the presence of sunlight. Reducing nitrogen oxide emissions is crucial for improving air quality and protecting human health.
USGS Volcanic Air Pollution Report: When Was It Written?
You may want to see also

Ozone pollution is especially harmful to people with asthma, metabolic disorders, and lung diseases
Ozone is a colourless gas made up of three oxygen atoms. It is formed through chemical reactions between natural and man-made emissions of nitrogen oxides (NOx) and volatile organic compounds (VOCs) in the presence of sunlight. These gaseous compounds mix in the outdoor air, and when they interact with sunlight, ozone is formed.
Ozone air pollution at ground level is harmful to human health. It aggressively attacks lung tissue by chemically reacting with it. It can cause the muscles in the airways to constrict, trapping air in the alveoli, which leads to wheezing and shortness of breath. It can also cause coughing and a sore or scratchy throat, and make it difficult to breathe deeply. These effects can be more serious in people with pre-existing lung diseases such as asthma, emphysema, and chronic bronchitis.
People with asthma are a large and growing segment of the population and are especially susceptible to the effects of ozone exposure. Children with asthma may use their inhalers more frequently on days when ozone concentrations are high. Long-term exposure to ozone is likely to be one of the many causes of asthma development. This is because ozone exposure can induce injury, inflammation, and increased airway reactivity, which may result in a worsening of a person's underlying asthma status, increasing the probability of an asthma exacerbation or a requirement for more treatment.
Ozone pollution is also especially harmful to people with metabolic disorders. Long-term exposure to ozone is associated with increased metabolic disorders, nervous system issues, and reproductive issues. Research also warns that breathing in other pollutants such as sulfur dioxide and nitrogen oxide can make the lungs react more strongly to ozone.
Vero Beach's Air Pollution Solutions
You may want to see also

Ozone is one of the six common air pollutants identified in the Clean Air Act
Ozone is a colourless gas made up of three oxygen atoms. It is one of the six common air pollutants identified in the Clean Air Act. The Clean Air Act (CAA) is a comprehensive federal law that regulates all sources of air emissions.
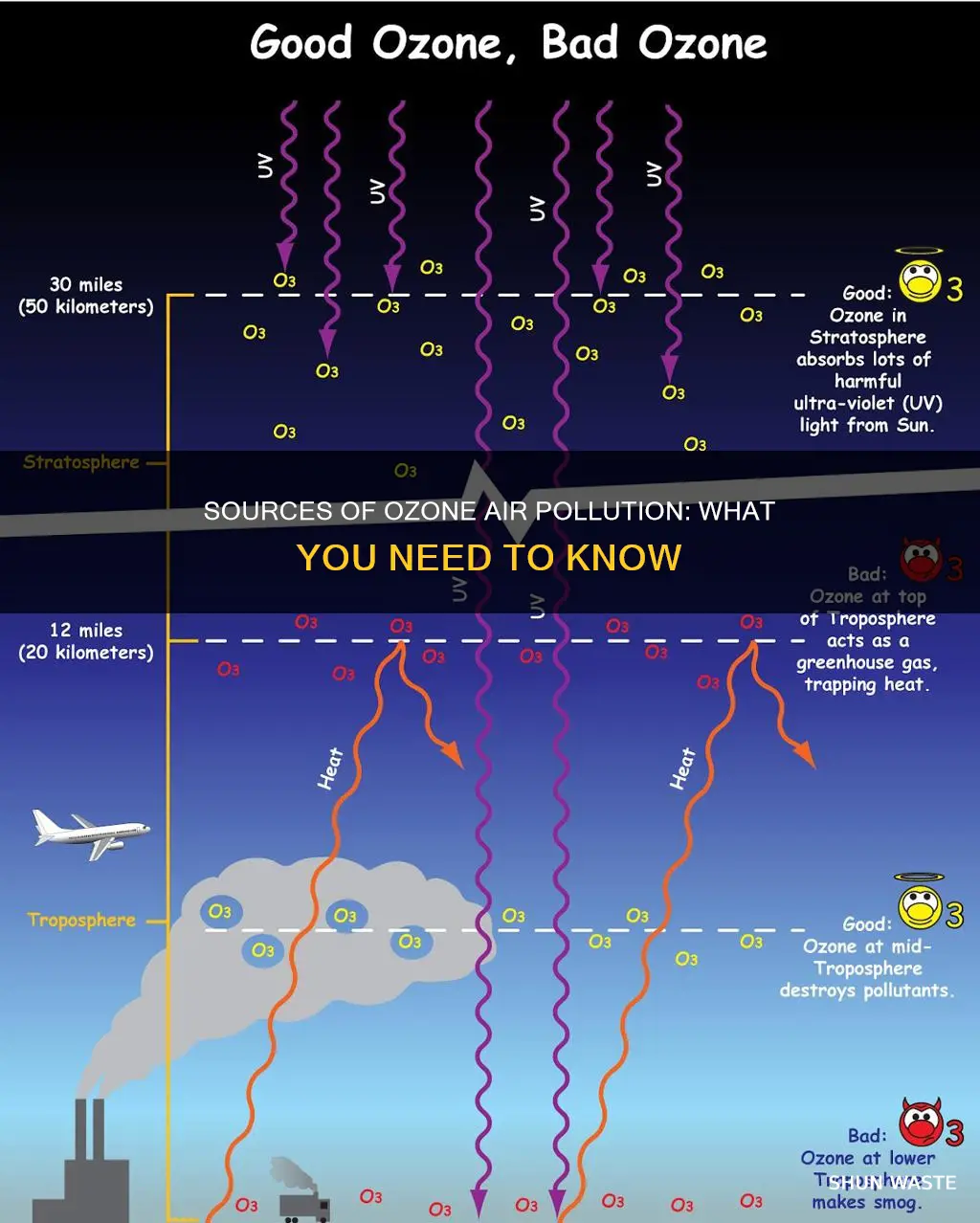
Ozone can be split into two major types: stratospheric ozone and ground-level ozone. Stratospheric ozone, also known as the "ozone layer", forms between 6 and 30 miles above the Earth's surface. It is formed when intense sunlight causes oxygen molecules (O2) to break up and reform as ozone molecules (O3). Stratospheric ozone forms a protective layer that shields us from the sun's harmful ultraviolet rays.
In contrast, ground-level ozone is formed just above the Earth's surface, up to about 2 miles above the ground. Ground-level ozone is not emitted directly into the air but is created by chemical reactions between oxides of nitrogen (NOx) and volatile organic compounds (VOCs) in the presence of sunlight. These chemical reactions occur due to pollutants emitted by cars, power plants, industrial boilers, refineries, and chemical plants, among other sources. Ground-level ozone is an irritant that negatively affects human health and the environment, earning it the label of "bad ozone". It is the main ingredient in smog and can cause respiratory issues, especially for individuals with asthma or other pre-existing respiratory conditions.
To address ground-level ozone pollution, the Environmental Protection Agency (EPA) has implemented various programs under Title VI of the Clean Air Act. These programs include the ODS Phaseout Program, which bans the production and import of ozone-depleting substances (ODS), and the Significant New Alternatives Policy (SNAP) Program, which identifies substitutes for ODS. The EPA also enforces regulations for managing ODS, such as the Motor Vehicle Air Conditioning Program and the Halons Program, which aim to reduce emissions and protect the ozone layer.
Air Pollution Pods: A Breath of Fresh Air?
You may want to see also

Ozone is an invisible, odourless gas, and the most widespread pollutant in the US
Ozone is a colourless, odourless gas made up of three oxygen atoms. It is naturally present in the upper atmosphere, where it forms a protective layer that shields us from the sun's harmful ultraviolet rays. This is known as "good ozone" or stratospheric ozone. However, ozone at ground level is a harmful air pollutant and is considered "bad ozone". Ground-level ozone is formed through chemical reactions between oxides of nitrogen (NOx) and volatile organic compounds (VOCs) in the presence of sunlight. These gaseous compounds mix in the ambient air, and when they interact with sunlight, ground-level ozone is formed.
Ground-level ozone is a significant concern in the United States, as it is the most widespread pollutant in the country. It is not emitted directly into the air but is a byproduct of various human activities. Sources of ground-level ozone pollution include emissions from cars, power plants, industrial boilers, refineries, chemical plants, paints, cleaners, solvents, and even lawn equipment. As a result, levels of ground-level ozone tend to be highest near urban centres.
The health effects of breathing air containing ground-level ozone are well-documented. Ozone can irritate and damage the tissues of the respiratory tract, causing inflammation, coughing, chest tightness, and worsening of asthma symptoms. It can also increase the permeability of lung cells, making them more susceptible to toxins and microorganisms. Both short-term and long-term exposure to ozone pollution have been linked to adverse health outcomes. Studies have shown that even healthy adults can experience greater obstruction of their airways when exposed to high levels of ozone. Certain groups are especially vulnerable to the effects of ozone, including people with pre-existing medical conditions such as asthma, metabolic disorders, and lung diseases. Children, older individuals, and those with respiratory issues are also considered sensitive groups.
The environmental impact of ground-level ozone pollution is also significant. Elevated ozone levels can affect sensitive vegetation and ecosystems, including forests, parks, wildlife refuges, and wilderness areas. Ozone can harm vegetation during the growing season and cause substantial damage to crops, native plants, and trees. Additionally, ozone can damage materials such as rubber and plastics.
Recognising the harmful effects of ground-level ozone, the Environmental Protection Agency (EPA) has implemented various measures to reduce emissions of pollutants that contribute to its formation. These include vehicle and transportation standards, regional haze and visibility rules, and regular reviews of national air quality standards. The EPA designates areas as attainment or nonattainment based on whether they meet these standards. By working with state and local governments, the EPA aims to improve air quality and protect public health and the environment from the detrimental effects of ground-level ozone pollution.
Air Pollution: The Invisible Danger?
You may want to see also
Frequently asked questions
Ozone air pollution, or "bad ozone", is formed from chemical reactions between oxides of nitrogen (NOx) and volatile organic compounds (VOCs) in sunlight. It is a harmful air pollutant due to its effects on people and the environment.
Ozone air pollution comes from pollution emitted by cars, power plants, industrial boilers, refineries, chemical plants, paints, cleaners, solvents, and motorized lawn equipment. Therefore, levels of ozone air pollution tend to be the highest near urban centres.
People at greatest risk of harm from breathing air containing ozone include people with asthma, lung diseases, metabolic disorders (e.g. obesity), and pre-existing medical conditions. Children and adults who spend more time outdoors participating in vigorous physical activities are also at greater risk.







