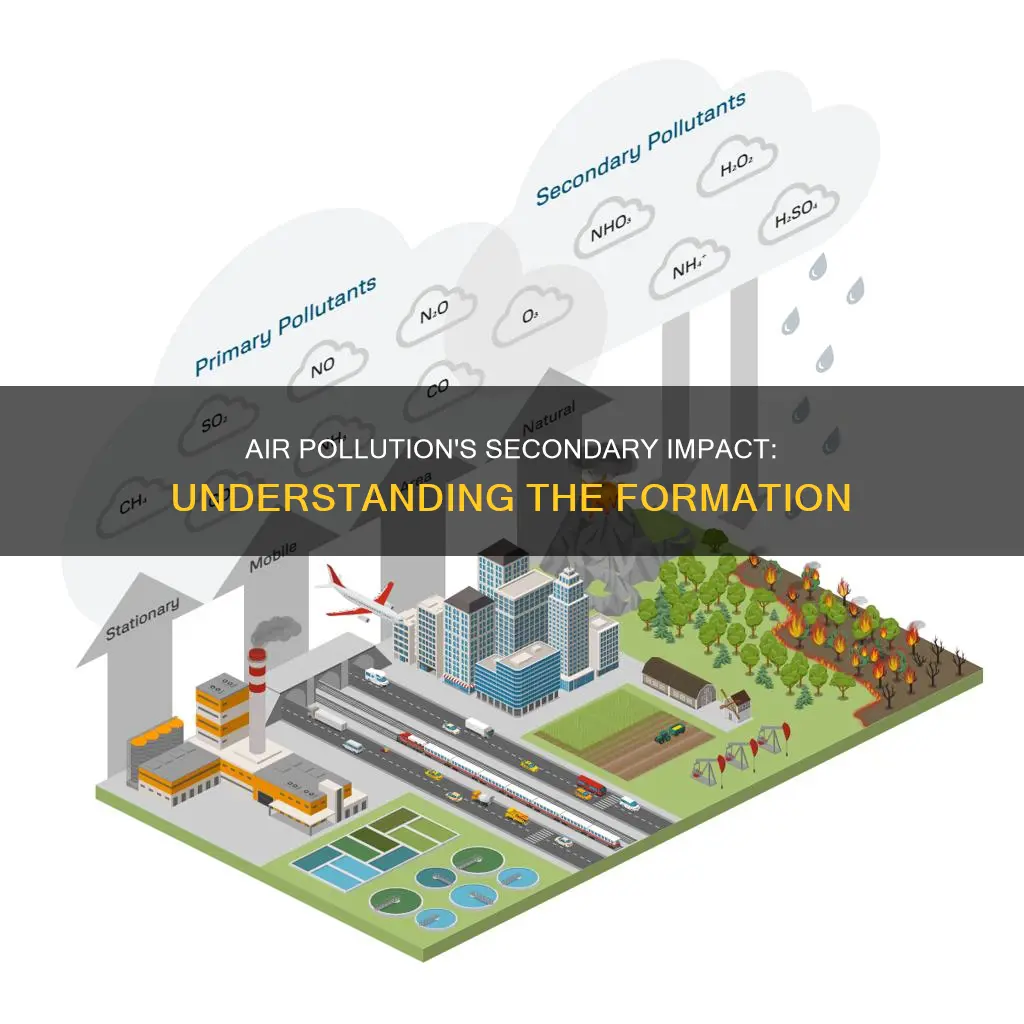
Secondary air pollutants are formed when primary pollutants, such as nitrogen dioxide, sulfur dioxide, and black carbon, react with other chemicals in the atmosphere. These reactions produce harmful compounds such as ozone, particulate matter, and acid rain, which can have detrimental effects on both human health and the environment. For example, acid rain can irritate the airways and eyes, and corrode important cultural structures. The formation of secondary air pollutants is highly dependent on local climatic factors, such as weather patterns and wind conditions, which influence the interaction and transformation of primary pollutants.
| Characteristics | Values |
|---|---|
| Formation | Sunlight reacts with NO2, which then interacts with other molecules in the air to form smog |
| Composition | Gaseous particles, including ground-level ozone, nitrogen dioxide, sulfur dioxide, and volatile organic compounds (VOCs) |
| Health Effects | Irritation of the airway and eyes, superficial decay of monuments and cultural structures |
| Sensitivity | Weather patterns, prominent in cities with warm and dense atmospheres |
What You'll Learn
- Nitrogen dioxide and other nitrogen oxides react with other chemicals to form secondary pollutants
- Black carbon, a major component of soot, is a secondary pollutant that is harmful to human health
- Photochemical smog is a secondary pollutant formed when sunlight reacts with nitrogen dioxide
- Acid rain is formed through interactions between nitrogen dioxide, sulfur dioxide, water, oxygen, and other atmospheric chemicals
- Particulate matter and ozone are formed when nitrogen dioxide and volatile organic compounds react

Nitrogen dioxide and other nitrogen oxides react with other chemicals to form secondary pollutants
Nitrogen dioxide (NO2) is a foul-smelling gas that is one of a group of gases known as oxides of nitrogen (NOx). Other nitrogen oxides include nitric oxide (NO), nitrous oxide, nitrous acid, and nitric acid. NO2 is formed through the high-temperature oxidation of diatomic nitrogen found in combustion air. This typically occurs at temperatures above 1300°C (2600°F).
NO2 and other nitrogen oxides can react with other chemicals in the air to form secondary pollutants. These reactions are sensitive to weather patterns and can be influenced by sunlight, heat, and other atmospheric conditions. One such reaction is between NO2 and volatile organic compounds (VOCs), which are compounds with carbon that easily become vapors or gases. This reaction produces ozone (O3), a major component of smog. Smog is a significant form of air pollution and can have adverse effects on human health, particularly for children, people with lung diseases, and those who work or exercise outside.
Another secondary pollutant formed from NO2 and other nitrogen oxides is particulate matter. These are tiny solid or liquid particles that can be suspended in the air and are harmful when inhaled due to their effects on the respiratory system. Acid rain is also a secondary pollutant that can be formed when NOx gases react with other chemicals in the atmosphere. This can occur through the oxidation of NOx to produce nitric acid (HNO3), which can then be precipitated as acid rain.
The formation of these secondary pollutants is influenced by various factors, including the concentration of NO2 and other nitrogen oxides, the presence of other chemicals in the atmosphere, and weather conditions such as temperature and solar radiation levels. Nitrogen oxides are emitted from vehicles, power plants, and other sources of fuel combustion, as well as natural sources such as lightning.
Burning Cardboard: Polluting the Air?
You may want to see also

Black carbon, a major component of soot, is a secondary pollutant that is harmful to human health
Secondary air pollutants are formed when primary pollutants interact with other molecules in the air. For instance, sunlight reacts with NO2, which then interacts with other molecules in the air to form smog. Black carbon is a major component of soot and is formed by the incomplete combustion of fossil fuels, wood, and biomass. It is considered a secondary pollutant and is harmful to human health.
Black carbon is a significant contributor to air pollution and has adverse effects on human health. It is produced by the burning of fossil fuels, wood, and other biomass fuels, as well as waste. Diesel-driven combustion engines, residential burning of wood and coal, power stations using heavy oil or coal, and field burning of agricultural wastes are the main sources of black carbon emissions. These sources vary across regions, with solid fuels and kerosene used for cooking, lighting, and heating contributing to nearly half of global anthropogenic black carbon emissions.
Black carbon is a complex mixture of gases and particulate matter, often referred to as soot. It contains fine, carcinogenic particles that are small enough to enter the bloodstream and reach other organs. Exposure to black carbon has been linked to cardiovascular diseases, including acute myocardial infarction and cardiovascular hospital admissions. It is also associated with the activation of implantable cardioverter defibrillators and an increased risk of cardiovascular hospitalization.
Additionally, black carbon may operate as a universal carrier of a wide variety of chemicals, including combustion-derived constituents, of varying toxicity to the human body. It can affect sensitive targets such as the lungs, defence cells, and possibly the systemic blood circulation. Reducing people's exposure to particulate matter containing black carbon is crucial to mitigating its health effects.
To address the harmful impacts of black carbon, several measures have been proposed. These include adopting clean cookstoves, reducing open waste burning, implementing clean cooking fuels and technologies, and developing policies for soot-free buses. These strategies not only improve human health but also contribute to slowing near-term climate warming and increasing crop yields.
Human Activities and Their Air Pollution Impact
You may want to see also

Photochemical smog is a secondary pollutant formed when sunlight reacts with nitrogen dioxide
Secondary air pollutants are formed when primary pollutants interact with other compounds in the atmosphere. Photochemical smog is a prominent example of a secondary air pollutant. It is formed when sunlight reacts with nitrogen dioxide (NO2) and other nitrogen oxides (NOx) in the atmosphere. This reaction occurs when ultraviolet light from the sun interacts with these compounds, which are emitted from the combustion of fossil fuels by vehicles, power plants, and factories.
Photochemical smog is a significant issue in densely populated cities with warm, dry climates, such as Los Angeles, Sydney, Mexico City, and Beijing. The landscape of these cities can also contribute to the buildup of smog. For example, Los Angeles is situated in a basin surrounded by mountains, trapping the smog in the valley.
The adverse effects of photochemical smog on human health are well-documented. Long-term exposure to the particulate matter in photochemical smog can lead to reduced lung function, heart attacks, irregular heartbeats, and premature deaths for individuals with heart and lung disease. It can also cause eye irritation and respiratory ailments. In addition, the ground-level ozone created by photochemical smog has been shown to damage crops and native plants, impacting entire ecosystems.
Nitrogen oxides (NOx) and volatile organic compounds (VOCs) are the two primary pollutants that react with sunlight to form photochemical smog. VOCs are released from gasoline, paints, and cleaning solvents. When these pollutants react with sunlight, they create ground-level ozone and air particulates, the main ingredients in photochemical smog.
As the urban population continues to grow globally, the problem of photochemical smog is expected to increase. This is particularly concerning given the adverse health and environmental effects of this secondary pollutant.
Air Pollution: Fuels and Their Harmful Emissions
You may want to see also

Acid rain is formed through interactions between nitrogen dioxide, sulfur dioxide, water, oxygen, and other atmospheric chemicals
Acid rain is a term used to describe any form of precipitation that contains high levels of nitric and sulfuric acids. This can include rain, snow, fog, hail, or even dust. Acid rain is formed through interactions between nitrogen dioxide, sulfur dioxide, water, oxygen, and other atmospheric chemicals.
Nitrogen dioxide (NO2) and sulfur dioxide (SO2) are primary air pollutants that are released into the atmosphere through human activities such as burning fossil fuels and industrial processes. Once in the atmosphere, these gases can undergo chemical reactions, leading to the formation of acid rain.
The process begins with the emission of nitrogen dioxide and sulfur dioxide from sources such as coal-burning power plants, factories, and automobiles. These gases then react with water vapour, oxygen, and other atmospheric chemicals. The specific chemical reactions involved in the formation of acid rain are complex and involve multiple steps.
Nitrogen dioxide, in the presence of sunlight, can react with water and oxygen to form nitrogen oxides (NOx), which then undergo further reactions to form nitric acid (HNO3). Similarly, sulfur dioxide can react with oxygen and water to form sulfur trioxide (SO3), which subsequently reacts with water to form sulfuric acid (H2SO4). These acids then mix with precipitation, resulting in acid rain.
The acids in acid rain can have far-reaching effects on the environment. When acid rain falls on the Earth's surface, it can damage ecosystems, including lakes, streams, wetlands, and other aquatic environments, making them more acidic. This increased acidity can lead to higher aluminium absorption from the soil, which is then carried into water bodies, creating toxic conditions for aquatic life. Additionally, acid rain can harm vegetation, buildings, and other physical structures, as well as cause health issues in humans when inhaled.
Factory Farms: Air Pollution Reporting: Who's Responsible?
You may want to see also

Particulate matter and ozone are formed when nitrogen dioxide and volatile organic compounds react
Nitrogen dioxide (NO2) is a reddish-brown gas that is soluble in water and a strong oxidant. It is formed from the high-temperature combustion of fuels used for heating, transportation, industry, and power generation. NO2 is a precursor to ground-level ozone, a major component of smog. Ground-level ozone is formed through chemical reactions between nitrogen oxides (NOx) and volatile organic compounds (VOCs) emitted from vehicles, power plants, industrial boilers, refineries, and chemical plants. These chemical reactions occur in the presence of sunlight, and the ozone peaks during the afternoon when sunlight is most intense.
Ozone (O3) is a highly reactive gas composed of three oxygen atoms. While stratospheric ozone is beneficial as it shields life from harmful ultraviolet radiation, ground-level ozone is detrimental to health. People with asthma, children, and the elderly are particularly vulnerable to the adverse effects of ground-level ozone.
Particulate matter (PM) refers to inhalable particles composed of sulphate, nitrates, ammonia, sodium chloride, black carbon, mineral dust, or water. PM can vary in size, with PM2.5 and PM10 being the most common in the regulatory framework and relevant for health. Particulate matter, like ozone, is formed when NO2 reacts with other chemicals in the air.
The formation of particulate matter and ozone through the reaction of NO2 with other air chemicals is a critical process in the creation of secondary air pollutants. These secondary pollutants are highly sensitive to weather patterns, with smog formation being influenced by sunlight interacting with NO2 and other atmospheric molecules.
Sources of Air Pollution: The Worst Offenders
You may want to see also
Frequently asked questions
Secondary air pollutants are formed when primary pollutants like nitrogen dioxide, sulfur dioxide, water, oxygen, and other chemicals interact to form new, harmful compounds.
Secondary air pollutants are formed through chemical reactions between primary pollutants. For example, sunlight reacting with nitrogen dioxide (NO2) can form smog, which is a secondary pollutant. NO2 can also react with volatile organic compounds to form particulate matter and ozone, both of which are harmful when inhaled.
Some examples of secondary air pollutants include smog, ozone, particulate matter, acid rain, and other toxic chemicals.







