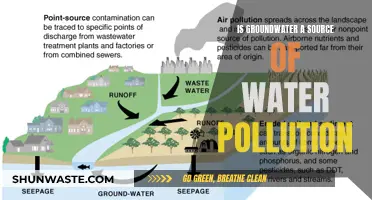
Water acidity is a significant contributor to pollution, with far-reaching consequences for the environment and human health. Acid rain, a well-known example of human influence on water pH, occurs when emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx) react with water, oxygen, and other chemicals in the atmosphere. The resulting sulfuric and nitric acids mix with water and fall to the earth as precipitation, leading to the acidification of lakes, rivers, and streams. This process has detrimental effects on aquatic ecosystems, harming fish, plants, and insects, and can also impact human health through the inhalation of fine sulfate and nitrate particles. Additionally, agricultural practices, wastewater discharge, and industrial runoff contribute to water pollution and pH fluctuations, further exacerbating the issue of water acidity and its impact on the environment.
| Characteristics | Values |
|---|---|
| How acidity contributes to water pollution | The pH level of water bodies decreases due to acid rain, which is caused by the emission of SO2 and NOx into the atmosphere, forming sulfuric and nitric acids. |
| The recommended pH range for most fish is between 6.0 and 9.0. | |
| Acid rain also contains nitrogen, which is responsible for declining fish populations and coral survival. | |
| Nitrogen and phosphorus in water or air can cause nutrient pollution, leading to algal blooms that are harmful to people and wildlife. | |
| Acidic water affects aquatic plants and insects, simplifying the entire aquatic food chain and making it less resilient and productive. | |
| Acids can enter water bodies through atmospheric diffusion from coal burning, acid rain, or volcanic activity. | |
| Dry deposition of acidic particles and gases can also occur, harming plants and wildlife when washed off by rainfall. | |
| Agricultural runoff, wastewater discharge, and industrial operations can increase or decrease water pH depending on the chemicals involved, contributing to water pollution. |
What You'll Learn

How does water acidity affect aquatic life?
Water acidity is a significant contributor to pollution, and it has detrimental effects on aquatic ecosystems. Acid rain, resulting from the emission of sulphur dioxide (SO2) and nitrogen oxides (NOx) into the atmosphere, is a primary driver of water acidity. These acidic particles fall as wet and dry deposition, impacting lakes, streams, and other water bodies. While acid rain itself is not harmful to humans, the pollutants that cause it can be. The ecological consequences of acid rain are most evident in aquatic environments, where it poses a threat to fish and other wildlife.
The effects of water acidity on aquatic life are far-reaching and complex. As water becomes more acidic, it can have a detrimental impact on the ability of shellfish and other species to build shells. This is particularly evident in oysters, where increased water acidity has resulted in massive die-offs in the Pacific Northwest of the United States. Mussels also struggle to cling to rocks effectively in acidic water, and their byssal threads are weakened. Additionally, acid rain can contribute to the accumulation of aluminium in water bodies, which can be harmful to various organisms and species.
The pH level of water is a critical factor in determining the health of aquatic ecosystems. A lower pH indicates higher acidity, which can be harmful to fish and other aquatic organisms. Not all fish and wildlife can tolerate the same levels of acidity, and a change in pH can impact their growth, reproduction, and chemical communication. This disruption in the natural balance of aquatic life can have far-reaching consequences for the entire food chain, including humans who depend on these water sources for food and economic activities such as fisheries and aquaculture.
Furthermore, water acidity can have indirect effects on aquatic life by influencing the availability of nutrients and the presence of toxic substances. For example, nutrient pollution, caused by excess nitrogen and phosphorus, can lead to algal blooms that are harmful to both people and wildlife. The increase in acidity can also affect the ability of water bodies to store pollutants, including future carbon emissions, further exacerbating the problem. This is particularly concerning given that agriculture is the leading cause of water degradation globally, with fertilizers, pesticides, and animal waste contributing to the contamination of rivers, streams, and lakes.
The impact of water acidity on aquatic life is a pressing issue that requires urgent attention. By understanding the delicate balance of aquatic ecosystems and the sensitivity of organisms to changes in pH, we can better address the problem of water pollution and work towards mitigating the harmful effects of water acidity on the diverse range of species that depend on healthy aquatic environments.
Stopping Water Pollution: Reducing Plastic's Impact
You may want to see also

What is acid rain and how does it form?
Acid rain is a form of precipitation that contains harmful amounts of nitric and sulfuric acids. It is caused by emissions of sulfur dioxide (SO2) and nitrogen oxide (NOx) into the atmosphere, which react with water, oxygen, and other chemicals to form acid rain. These emissions primarily come from the burning of fossil fuels, with smaller contributions from natural sources such as volcanoes. Once formed, these acidic particles and gases can be transported long distances by wind and air currents before being deposited onto surfaces through wet or dry deposition.
The formation of acid rain begins with the release of SO2 and NOx gases into the atmosphere, primarily from the burning of fossil fuels. These gases then undergo oxidation, reacting with water, oxygen, and other chemicals to form sulfuric acid and nitric acid, respectively. The chemical reactions involved in this process are:
2SO2 (g) + O2 (g) + 2H2O (l) → 2H2SO4 (aq)
4NO2 (g) + O2 (g) + 2H2O (l) → 4HNO3 (aq)
Acid rain typically has a pH value between 4.2 and 4.4, significantly lower than the pH of regular rainwater, which is around 5.6 to 5.7. This lower pH makes acid rain harmful to the environment and human health. It can cause damage to forests, freshwater ecosystems, soils, and buildings, as well as harm plants, insects, and aquatic life. Additionally, when acid rain falls on agricultural lands, it can alter the composition of the soil and wash away essential nutrients required for plant growth.
The ecological consequences of acid rain are most evident in aquatic environments, such as streams, lakes, and marshes. As acid rain flows through the soil and into these water bodies, it can leach aluminum from soil clay particles, increasing the aluminum concentration in the water. This elevated aluminum content, along with the lower pH, can be detrimental to fish and other aquatic organisms, impacting their survival and reducing biodiversity.
To address the issue of acid rain, it is crucial to transition to renewable and alternative energy sources, such as solar, wind, nuclear, hydropower, and geothermal energy. By reducing emissions of sulfur dioxide and nitrogen oxide, we can mitigate the formation of acid rain and minimize its detrimental effects on the environment and human health.
Fabric Industry Discharge: Water Pollution's Hidden Danger
You may want to see also

How does water acidity impact human health?
Water acidity is a significant contributor to pollution, and it has a direct impact on human health. While walking in acid rain or swimming in an acidic lake is not considered dangerous to humans, the pollutants that cause acid rain, such as SO2 and NOx, can be harmful when inhaled. These pollutants can lead to respiratory issues, particularly for individuals with asthma, and have been linked to adverse effects on heart function. Additionally, NOx emissions contribute to ground-level ozone, which poses risks to human health.
The impact of water acidity on human health is also evident through its effect on drinking water sources. Acidic water, with a pH below 7, often contains high levels of heavy metals, including lead, arsenic, copper, nickel, cadmium, chromium, and zinc. Regular consumption of acidic water with these contaminants can lead to heavy metal poisoning and toxicity, posing a severe threat to human health. The presence of heavy metals in drinking water can also have detrimental effects on bone health, and there are concerns about the potential for acidic water to prevent calcium absorption, leading to bone loss over time.
Acidic water is not suitable for drinking due to its high concentration of heavy metals and potential health risks. One of the primary concerns is the increased likelihood of heavy metal leaching from the environment into the water, resulting in a higher concentration of these toxic substances. Prolonged exposure to heavy metals in drinking water can lead to serious illnesses, especially in children, including organ damage, immune system suppression, and respiratory issues. Additionally, the high acidity of the water can corrode metal pipes, further increasing the presence of heavy metals in the water supply.
While the United States Environmental Protection Agency (EPA) does not regulate the pH of drinking water, it recommends maintaining a pH range of 6.5 to 8 for public water supplies. The EPA also monitors aquatic ecosystem health and the response of water bodies to changes in acid-causing emissions through its Long-Term Monitoring (LTM) Network, which collects data from over 280 sites.
In addition to the direct health impacts, water acidity also contributes to environmental pollution, particularly in aquatic ecosystems. Acid rain, a significant contributor to water acidity, can harm fish, shellfish, and other wildlife in streams, lakes, and marshes. It can also affect plant life, as it leaches aluminum from the soil, removing essential minerals and nutrients needed for growth. This disruption in one part of the ecosystem can have a ripple effect on other organisms, demonstrating the interconnectedness within these environments.
Urban Water Pollution: Sources and Solutions
You may want to see also

How does water acidity affect the soil?
Water acidity can have a significant impact on soil, influencing both its chemical composition and the ability to support plant life. Soil pH, which measures acidity or alkalinity, plays a crucial role in determining the availability of essential nutrients for plants. A pH of 7 is considered neutral, with lower values indicating acidity and higher values indicating alkalinity.
Soil acidity can occur naturally due to factors such as rainfall amount and intensity, the chemical composition of parent materials, and the presence of decaying organic matter. Sandy soils, for instance, tend to become acidic faster due to the rapid percolation of water and their lower content of clay and organic matter. Additionally, soils developed from granite are typically more acidic than those originating from calcareous shale or limestone.
The effects of water acidity on soil are particularly evident in regions with thin soil layers, such as the mountainous areas of the Northeast United States. In these areas, the soil struggles to neutralize acidic rainwater, leading to the accumulation of acid and aluminium in the soil, streams, or lakes. This phenomenon, known as episodic acidification, can cause short-term stress on the ecosystem, potentially injuring or killing various organisms and species.
Acidic soils can negatively impact plant productivity by decreasing the availability of essential nutrients like phosphorus, iron, and molybdenum. Moreover, high levels of aluminium and manganese in acidic soils can become toxic to plants, hindering their growth and even leading to crop failure. Liming is a common practice used to raise the pH of acidic soils, making nutrients more available for plant uptake and improving agricultural productivity.
Water acidity also contributes to pollution by carrying pollutants from the atmosphere to the soil. Acid rain, formed by the reaction of sulphur dioxide (SO2) and nitrogen oxides (NOx) with water, oxygen, and other chemicals, can deposit acids onto the soil through wet and dry deposition. These acidic particles can harm soil organisms, degrade the environment for bacteria and earthworms, and negatively affect plant growth by impeding root development.
Waterways: Pollutants' Unseen Journey and Impact
You may want to see also

How does water acidity contribute to pollution in agricultural areas?
Water acidity contributes to pollution in agricultural areas in several ways, and this has a significant impact on the environment and ecosystems. Firstly, it is important to understand that water with a pH below 7 is considered acidic, and water in this range is often used for irrigation. The pH level of water is crucial as it can affect the growth and health of plants. Water with high alkalinity, for example, can alter the pH of the growing medium, interfering with nutrient uptake and causing deficiencies that harm plant health.
Agricultural practices can increase water acidity and, subsequently, pollution in a few ways. The use of pesticides and chemical fertilizers is a major contributor. When these substances are applied to crops, they can run off into nearby water sources, increasing the acidity and toxicity of the water. This is particularly harmful to aquatic life, as certain pH levels can prevent fish eggs from hatching and even kill adult fish. The presence of pesticides and fertilizers in water can also indicate the contamination of water sources, and this has been observed in surface water and groundwater.
Another way in which water acidity is increased in agricultural areas is through the use of manure. Livestock and poultry manure can contain bacteria and excess nutrients, which, when washed into water sources, can cause harmful algal blooms and hypoxic conditions that are detrimental to aquatic life. This is a concern as it can affect drinking water supplies and the health of humans and wildlife.
Agricultural pollution also comes in the form of veterinary medicines, such as antibiotics, which can enter water sources and impact downstream ecosystems. Furthermore, the intensive farming of animals and crops has led to an increase in fish excreta and uneaten feed, which diminishes water quality.
The impact of water acidity and pollution in agriculture is far-reaching. It affects not only the immediate environment and aquatic life but also human health and food production. To mitigate these issues, various techniques can be employed, such as buffer strips of vegetation along farm margins and rivers, and integrated farming systems that optimise resource use and reduce pollution.
Purifying Polluted Water: Effective Filtration Techniques Explored
You may want to see also
Frequently asked questions
Water acidity refers to the pH level of a water body, which can range from 0 to 14. A pH level of 7 is considered neutral, with anything below 7 being acidic and above 7 being alkaline. Most natural water sources have a slightly acidic pH of around 6. However, due to various human activities and natural processes, the pH level of water can fluctuate, leading to increased acidity or alkalinity.
Water acidity can contribute to pollution in several ways. Firstly, human activities such as industrial emissions, agricultural runoff, and wastewater discharge can release acids or alkaline substances into water bodies, altering their pH levels. For example, acid rain, caused by the emission of sulphur dioxide (SO2) and nitrogen oxides (NOx) from burning fossil fuels, can acidify lakes, streams, and rivers. Additionally, agricultural practices can contribute to nutrient pollution, with excess nitrogen and phosphorus leading to algal blooms that are harmful to both people and wildlife.
Increased water acidity can have significant ecological impacts. It can harm aquatic life, including fish, shellfish, insects, and plants, by making it difficult for them to survive and reproduce. This disruption can simplify the entire aquatic food chain, reducing the health and resilience of the ecosystem. Additionally, water acidity can corrode metals and damage buildings and monuments. It can also impact human health by affecting heart and lung function when inhaled.