Smog is a type of air pollution that reduces visibility and significantly affects air quality. It is formed when certain pollutants in the air react chemically under the influence of sunlight. These pollutants are emitted from fossil fuel-powered vehicles, industries, and other combustion sources. Smog can irritate the eyes and throat and damage the lungs, especially for children, senior citizens, and people with asthma or allergies. It also has negative effects on plants and the environment. Many countries have created laws and implemented programs to reduce smog and improve air quality, such as regulating emissions and investing in clean vehicle technology.
| Characteristics | Values |
|---|---|
| Definition | Air pollution that reduces visibility |
| Composition | Nitrogen oxides, sulfur oxide, ozone, smoke, and other particulates |
| Sources | Fossil fuel combustion, vehicular emissions, industrial emissions, forest and agricultural fires, and photochemical reactions |
| Health Effects | Eye and throat irritation, respiratory problems, exacerbation of asthma and other lung diseases, increased respiratory infections |
| Environmental Effects | Acidification of soil and water, deterioration of ecosystems, contribution to climate change |
| Measurement | Automated optical instruments, air quality indexes (e.g., American Air Quality Index, Malaysian API) |
| Regulations | US Clean Air Act, EPA standards and programs, International Maritime Organization (IMO) Emission Control Area (ECA) |
What You'll Learn
- Smog is caused by emissions from burning fossil fuels and reacting with sunlight
- Smog is a mixture of air pollutants that affect air quality and human health
- Photochemical smog is produced when nitrogen oxides and volatile organic compounds react
- Tropospheric ozone is a harmful gas that is a key component of smog and causes respiratory issues
- High humidity and poor air circulation accelerate chemical reactions that form smog

Smog is caused by emissions from burning fossil fuels and reacting with sunlight
Smog is a type of intense air pollution that reduces visibility and significantly affects air quality. The word "smog" was coined in the early 20th century, derived from the combination of the words "smoke" and "fog". It was used to describe the mix of smoke and fog that was common in industrial areas. Today, smog is still a familiar sight in some cities, particularly those with high levels of industry and traffic.
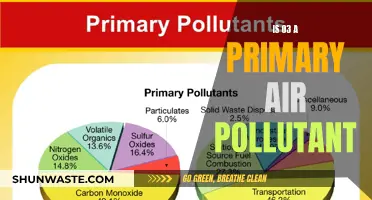
The main cause of smog is emissions from burning fossil fuels, such as coal, gasoline, and natural gas. These emissions react with sunlight to form airborne particles and ground-level ozone, also known as tropospheric ozone. Ozone is a key component of smog and is harmful to human health, causing respiratory problems and other ailments. It can irritate the eyes, nose, and throat and damage the lungs, especially in children, the elderly, and people with asthma or allergies.
Vehicular emissions from internal combustion engines, industrial emissions from factories and power plants, and forest and agricultural fires also contribute to smog formation. Smog is more common on sunny and hot days when solar radiation is more intense, and it is often worse in cities located in basins surrounded by mountains, as the smog can become trapped in the valley and unable to be carried away by the wind.
The effects of smog are not just limited to human health; it also damages the environment. Air pollutants contribute to the acidification of soil and water, affecting local flora and fauna. Smog can also reduce visibility, which can impact transportation and daily life.
To combat smog and improve air quality, many countries, including the United States, have implemented laws and standards to reduce emissions and regulate the release of chemicals into the atmosphere. The US Environmental Protection Agency (EPA), for example, has set standards for smog and created programs that lead to investments in clean vehicle and engine technology. These efforts have resulted in significant improvements in air quality and public health.
Air Pollution's Infant Mortality Link: What's the Truth?
You may want to see also

Smog is a mixture of air pollutants that affect air quality and human health
Smog is a type of air pollution that significantly affects air quality and human health. It is formed when certain pollutants in the air react chemically under the influence of sunlight. This process is more common on sunny and hot days when solar radiation is more intense. The primary sources of smog are cars, trucks, factories, power plants, and anything that combusts fossil fuels such as coal, gasoline, or natural gas.
The word "smog" was coined in the early 20th century to describe the mixture of smoke and fog that was common in industrial areas. Today, most of the smog we see is photochemical smog, which is produced when sunlight reacts with nitrogen oxides and volatile organic compounds (VOCs) in the atmosphere. VOCs are released from gasoline, paints, and cleaning solvents. When these chemicals react with sunlight, they form airborne particles and ground-level ozone, which is the main gas contributing to smog formation.
Ozone is a key component of smog and is notoriously harmful to human health. It can cause itchy, burning eyes, irritate the throat, and damage the lungs, especially in children, the elderly, and people with asthma or allergies. Prolonged exposure to ozone and other pollutants in smog can lead to serious respiratory problems and exacerbate diseases such as asthma, emphysema, and bronchitis. Smog also has negative effects on the environment, contributing to the acidification of soil and water and reducing the quality of life for inhabitants of highly polluted cities.
To address the harmful effects of smog, governments and organizations have implemented various programs and standards to reduce air pollution. For example, the United States Environmental Protection Agency (EPA) has set standards for smog and created programs that lead to investments in clean vehicle and engine technology. The Diesel Emissions Act Reduction program offers funding for projects that reduce harmful emissions from diesel engines. Similarly, the International Maritime Organization (IMO) has designated certain coastal areas as Emission Control Areas (ECAs), where large vessels must adhere to stricter emissions and fuel standards. These efforts to reduce emissions and improve air quality are expected to have significant benefits for public health and the environment.
Wood Burning: Air Pollution's Unseen Impact
You may want to see also

Photochemical smog is produced when nitrogen oxides and volatile organic compounds react
Photochemical smog is a type of air pollution caused by the interaction of sunlight with nitrogen oxides and volatile organic compounds (VOCs) in the atmosphere. Nitrogen oxides, commonly known as NOx, are produced by burning fossil fuels and are present in car exhaust, coal power plants, and factory emissions. VOCs, on the other hand, are released from gasoline, paints, and cleaning solvents.
When these pollutants are exposed to sunlight or ultraviolet radiation, they undergo a series of reactions that lead to the formation of photochemical smog. The nitrogen oxides can react with sunlight to produce singular oxygen atoms, which then combine with other oxygen atoms to form ozone. This ozone, when present at ground level, is harmful to human health and can irritate the eyes and throat and damage the lungs.
Additionally, nitrogen oxides can react with hydrocarbons, which are another type of VOC, to form other volatile compounds known as peroxyacetyl nitrate (PAN). The accumulation of ozone, PAN, and other volatile compounds, along with the energy from the sun, creates the brown haze of photochemical smog that is often observed on hot, sunny days in densely populated cities.
The largest contributors to photochemical smog are automobiles, as the combustion of fossil fuels in car engines releases significant amounts of nitrogen oxides and hydrocarbons into the atmosphere. Coal-fired power plants and other industrial sources also contribute to the necessary pollutants for photochemical smog formation.
To minimize photochemical smog levels, it is essential to reduce the use of fossil fuels and transition to non-polluting or sustainable sources of energy, such as nuclear power, hydropower, and wind power.
Strategies to Reduce Air Pollution in Cities
You may want to see also

Tropospheric ozone is a harmful gas that is a key component of smog and causes respiratory issues
Smog is a type of air pollution that reduces visibility and irritates the eyes, throat, and lungs. It is particularly harmful to children, senior citizens, and people with asthma or allergies. The term "smog" was first used in the early 1900s to describe a mixture of smoke and fog, which was common in industrial areas.
Tropospheric ozone is a key component of smog. It is a harmful gas that occurs naturally in the troposphere, the lowest level of the Earth's atmosphere. Tropospheric ozone is formed by chemical reactions between nitrogen oxides (NOx) and volatile organic compounds (VOCs) in the presence of sunlight. These chemicals are emitted by cars, power plants, factories, and other sources that combust fossil fuels.
The concentration of tropospheric ozone is typically between 20 and 30 parts per billion by volume (ppbv), but it can reach up to 100 ppbv in polluted areas. It is considered a harmful air pollutant due to its effects on human health and the environment. Ozone at ground level can irritate the eyes and throat, damage lung tissue, and trigger respiratory issues, especially in vulnerable individuals such as children, the elderly, and people with asthma.
Tropospheric ozone is also a greenhouse gas, contributing to global warming and climate change. It traps heat in the atmosphere, leading to warmer temperatures and the associated impacts of climate change, such as rising sea levels and more extreme weather. The levels of tropospheric ozone have been rising since the Industrial Revolution, particularly in urban areas with high emissions from vehicles and industries.
To reduce the production of tropospheric ozone, individuals can choose public transportation, walk, or bike instead of driving. Additionally, it is important to reduce the emissions of pollutants that lead to the formation of ozone, such as nitrogen oxides and volatile organic compounds. Governments and organizations, such as the US Environmental Protection Agency (EPA), have implemented programs and standards to reduce emissions and improve air quality, which have resulted in significant health benefits.
Air Pollution: Understanding the Causes and Effects
You may want to see also

High humidity and poor air circulation accelerate chemical reactions that form smog
Smog is a form of air pollution that results from the reaction of sunlight with pollutants such as nitrogen oxides and volatile organic compounds (VOCs) in the atmosphere. These reactions lead to the formation of a complex mixture of hundreds of different chemicals, including ground-level ozone (O3), a major component of smog.
Ozone is a colourless gas that, when found in the upper atmosphere, protects life on Earth from the sun's ultraviolet rays. However, ground-level ozone is a harmful air pollutant that can cause respiratory issues, irritate the eyes and throat, and damage the lungs, especially in children, the elderly, and those with asthma or allergies.
The formation of smog is influenced by various factors, including weather conditions and air circulation. High humidity, or relative humidity (RH), can accelerate the chemical reactions that produce smog. This is due to the increased water vapour content in the air, which facilitates the absorption of free oxygen molecules and accelerates the consumption of O3. Additionally, RH may influence O3 formation by impacting the generation of atmospheric OH radicals through HONO photolysis.
Poor air circulation and stagnant weather conditions hinder the dispersion of pollutants, leading to increased concentrations in the air. This, combined with high humidity, further enhances the likelihood of chemical reactions occurring and contributes to the formation of smog.
To combat smog formation, it is crucial to promote sustainable practices and reduce emissions from vehicles, industries, and power plants. Implementing measures such as improving public transport, using electric vehicles, and establishing low-emission zones in urban areas can significantly reduce the pollutants in the air and mitigate the formation of smog.
Global Warming's Air Pollution: A Complex Climate Concern
You may want to see also
Frequently asked questions
Smog is a type of intense air pollution. The term was coined in the early 20th century to describe a mix of smoke and fog. It is formed when certain pollutants in the air react chemically under the influence of sunlight.
Smog is derived from coal combustion emissions, vehicular emissions, industrial emissions, forest and agricultural fires, and photochemical reactions of these emissions. The main actors in the formation of smog are ozone (the main smog-forming gas), nitrogen oxides, and carbon monoxide.
Smog can cause itchy, burning eyes and irritate the throat. It can also damage the lungs, especially those of children, senior citizens, and people who work or exercise outdoors. Prolonged exposure to smog can lead to serious respiratory problems and exacerbate diseases such as asthma, emphysema, and bronchitis. In addition, air pollutants in smog contribute to the acidification of soil and water, affecting local flora and fauna.