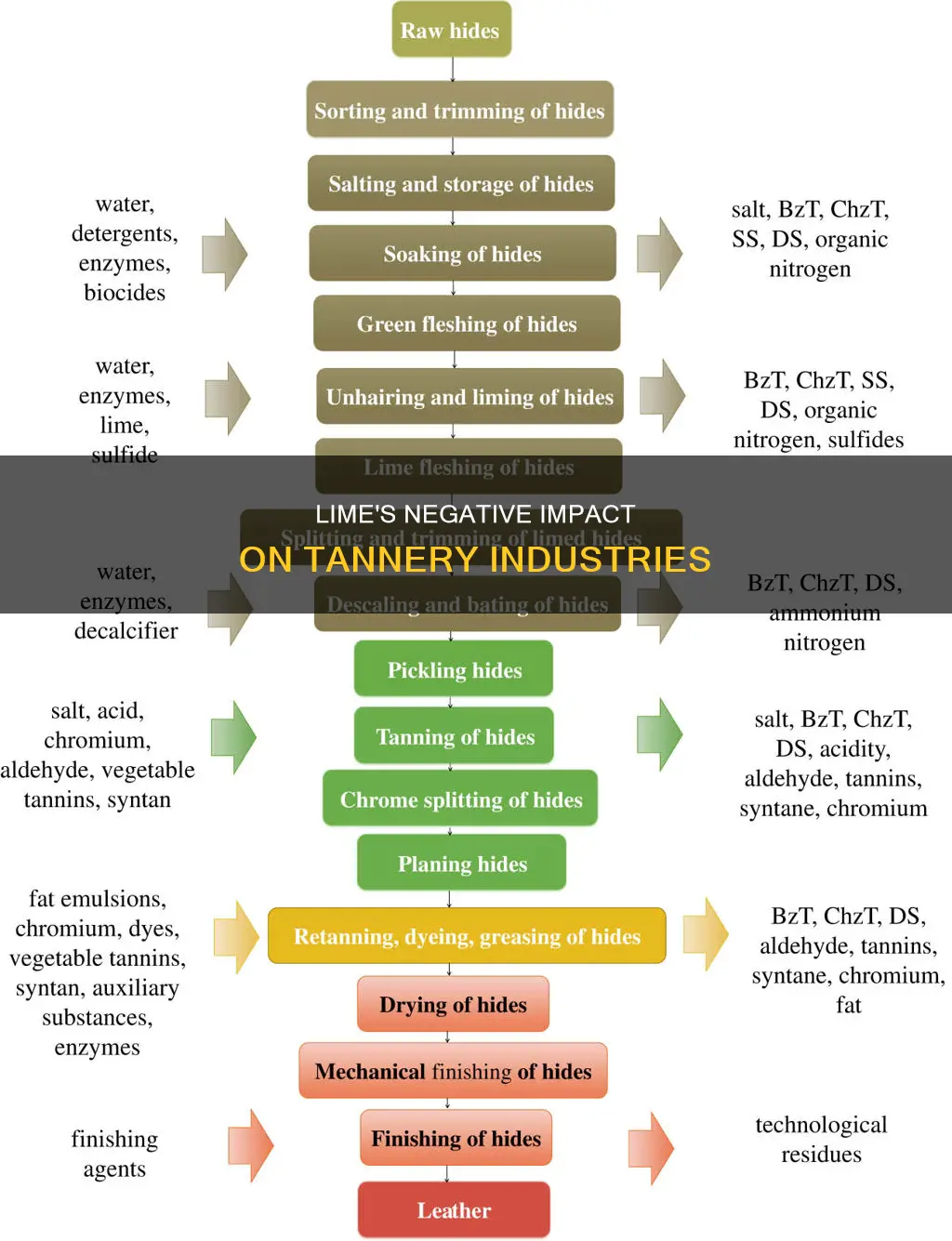
The tanning industry is responsible for a significant amount of water pollution, with untreated wastewater causing environmental issues and posing health risks. During the tanning process, around 300 kg of chemicals, including lime, are added per ton of hides. Lime is used in the unhairing process, which is a major source of pollution, as it produces large amounts of toxic sulfide and sludge. The sludge contains lime and chromium, which is harmful to the aquatic system and dangerous to human health. The high alkalinity of lime also poses chemical hazards, and can cause burns to the skin, eyes, nose, throat, and lungs. Therefore, it is important to address the pollution caused by lime in the tanning industry to mitigate environmental and health risks.
| Characteristics | Values |
|---|---|
| Lime's role in the tanning process | Lime is used in the tanning process to treat animal skins and hides and turn them into leather |
| Lime's impact on pollution | Lime contributes to pollution in tannery industries by creating large amounts of lime sludge and total solids formation |
| Environmental impact | Lime contributes to water pollution and causes toxic substances that lead to the death of living organisms |
| Health impact | Lime can cause chemical burns to the nose, throat, or lungs if inhaled in large volumes |
| Eye hazards | Lime splashes in the eyes can cause calcification of the cornea and loss of vision |
| Skin and eye protection | Working with lime requires the use of PPE such as helmets, gloves, and protective masks to prevent chemical burns |
What You'll Learn
- Lime is used in the chemical dissolution of hair and epidermis during the unhairing process
- Lime's low solubility can cause swelling and the formation of large amounts of sludge and total solids
- Lime is a disinfectant and emits no pollutants, but its use in construction can cause chemical burns
- Lime is a corrosive product with a high basic pH of 12 to 13, which can cause irreversible damage to the respiratory tract, skin, and eyes
- Lime is used in the treatment of tannery effluent to remove harmful chromium species from wastewater

Lime is used in the chemical dissolution of hair and epidermis during the unhairing process
The use of lime in the tanning industry has come under scrutiny due to its environmental impact. Lime is used in the chemical dissolution of hair and epidermis during the unhairing process, which is a heavy pollution operation. The conventional lime-sulfide process produces a large amount of sulfide, which is toxic and challenging to dispose of.
The unhairing process aims to remove the hair, epidermis, and to some degree, the inter-fibrillary proteins. It also prepares the hide for the removal of loose flesh and fat through the fleshing process. The use of lime and sodium sulfide can cause increased pH, biological oxygen demand (BOD), chemical oxygen demand (COD), and the generation of toxic sulfide gases, such as hydrogen sulfide.
Unhairing methods can be categorised into two groups: those that destroy or modify the epidermis tissue surrounding the hair, and those that attack the hair itself, destroying its structure. The use of lime falls into the second category, as it chemically dissolves the hair. One method involves the use of a drum unhairing process, where hides are soaked in a sodium sulfide solution. Lime is often added to this solution, and the hair and epidermis are reduced to a pulp that can be washed off.
To address the environmental concerns associated with lime usage, cleaner technologies and enzymatic processes have been developed. Enzymes can be used in combination with lower quantities of sodium sulfide and lime during the unhairing and liming processes. These enzymes attack the bond between the hair and the derma, leading to loose hair that can be easily recovered for other purposes, such as felt manufacturing, organic fertiliser, or poultry feed. Enzymatic processes offer shorter processing times, decreased chemical usage, and a cleaner grain layer on the final leather product.
Infectious Diseases: Water Pollution's Hidden Cause?
You may want to see also

Lime's low solubility can cause swelling and the formation of large amounts of sludge and total solids
The use of lime in the tanning industry has raised concerns about its environmental impact. During the tanning process, around 300 kg of chemicals, including lime, are added per ton of hides. Lime's low solubility can cause swelling and the formation of large amounts of sludge and total solids, which is a significant drawback.
Lime softening can have unintended consequences for biological systems, such as the removal of alkalinity and the creation of calcium carbonate (CaCO3) sludge. Pound for pound, lime produces more sludge than alternative products like Thioguard, which produce only water-soluble products with no added sludge. Lime can generate as much as 5 tons of 20% sludge cake per ton, contributing to the already high levels of pollution associated with the tanning industry.
The beamhouse, responsible for pre-tanning operations, is a major source of pollution, with over 60% attributed to liming and reliming processes. The use of lime, in combination with other chemicals, can lead to the formation of large amounts of sludge and total solids. This sludge contains toxic metals, which can limit its end use if concentrations exceed regulated limits. The high pH levels created by lime help retain these metals in the soil solids, preventing their uptake by plants.
To address the environmental concerns, there has been a push for cleaner tanning methodologies and a zero-waste concept. Recycling of unhairing lime-sulfide liquors has been proposed to reduce economic and environmental costs, as the conventional process produces large amounts of toxic sulfide. Additionally, the reduction of total water use and the minimization of chemicals, such as lime, salt, and sulfide, are considered essential to decreasing the pollution load from tanneries.
Lanterns: A Beautiful Tradition or Polluting the Environment?
You may want to see also

Lime is a disinfectant and emits no pollutants, but its use in construction can cause chemical burns
Lime is a key component in the leather tanning process, and its use in this context has raised environmental concerns. The tanning industry is known for its high pollution levels, particularly water pollution, and the use of lime contributes to this issue. During the tanning process, approximately 300 kg of chemicals, including lime, are added per ton of hides. This generates a significant amount of solid and liquid waste, which, if not properly treated, can cause severe environmental damage.
The tanning process involves several stages, including pre-tanning (or beam house operations), tanning or tanyard operations, and wet finishing or post-tanning. The beam house processes, in particular, are known for their uncleanness, with over 60% of the total pollution emerging from the liming-reliming processes. The use of lime, in combination with other chemicals like sodium sulphide, leads to the formation of large amounts of lime sludge and total solids, which are challenging to dispose of.
The environmental impact of lime in the tanning industry is further exacerbated by the presence of chromium in the waste. Chromium is a toxic substance used in the tanning process that, if discharged into water bodies without proper treatment, can cause severe ecological damage and pose risks to human health. The removal of chromium from tannery wastewater has been a focus of research, with methods such as using water hyacinth roots or brown seaweed to reduce chromium levels in the effluent.
While lime contributes to pollution in the tanning industry, it is important to note that lime itself is a disinfectant and does not emit pollutants. However, its use in construction and other industries can pose different health and safety risks, primarily in the form of chemical burns. Lime is commonly used in construction as a traditional masonry material, often in the form of cement. The high alkalinity of lime in cement makes it corrosive, and exposure can lead to chemical burns on the skin, eyes, nose, throat, or lungs.
To prevent chemical burns when working with lime in construction, it is crucial to wear personal protective equipment (PPE), such as helmets, gloves, and protective masks. Additionally, when mixing lime with water, it is important to follow safety protocols to avoid splashes that can cause thermal or chemical burns. Quicklime, for example, should be gradually poured into water and stirred throughout the operation to prevent splashing and ensure even heat distribution.
Nuclear Reactors: Pollution or Clean Energy?
You may want to see also

Lime is a corrosive product with a high basic pH of 12 to 13, which can cause irreversible damage to the respiratory tract, skin, and eyes
Lime is a highly corrosive substance, with a high basic pH of 12–13. This alkalinity is a significant factor in the chemical hazards it presents. While a brief exposure to lime causes little risk, more extended contact with dry or wet cement can cause irreversible damage to the respiratory tract, skin, and eyes.
Lime is an inorganic material composed primarily of calcium oxides and hydroxides. It is also the name for calcium oxide, which is used as an industrial mineral. Calcium oxide is made by heating calcium carbonate in a kiln to a temperature of over 900°C (1,650°F). This process converts the calcium carbonate into burnt lime, unslaked lime, or quicklime (calcium oxide).
Through the subsequent addition of water, the highly caustic and reactive burnt lime becomes the less caustic (but still strongly alkaline) slaked lime or hydrated lime (calcium hydroxide). This process is called slaking of lime. Slaked lime is commonly used as a binding mortar in masonry due to its adhesive properties with bricks and stones. It is also used in whitewashing as a wall-coat to help the whitewash adhere to the wall.
The corrosive nature of lime means that it can cause chemical burns to the skin, eyes, and respiratory tract. The severity of the injury will depend on the concentration and temperature of the lime, as well as the duration of exposure. When lime comes into contact with the eyes, it can cause anything from moderate irritation to chemical burns resulting in blindness. If lime is in powder form, moisture in the eyes will promote the penetration of the substance, and pain may appear hours after exposure when a significant injury has already occurred.
In the context of tannery industries, lime is used in the unhairing process, where it is combined with sulphide to chemically dissolve the hair and epidermis of animal skins. This process produces a large amount of sulphide, which is toxic and difficult to dispose of. The use of lime in this process is a significant source of pollution, and the lime sludge that is formed is a major drawback.
Radioactive Pollution: Mining's Cancer Risk?
You may want to see also

Lime is used in the treatment of tannery effluent to remove harmful chromium species from wastewater
The tanning industry is responsible for a significant amount of water pollution. During the tanning process, about 300 kg of chemicals, including lime, are added per ton of hides. Lime is used in the tanning process to remove hair and epidermis from animal skins or hides. However, the conventional lime–sulfide process produces a large amount of sulfide, which is toxic and challenging to dispose of. The use of lime also results in the formation of huge amounts of lime sludge and total solids, contributing to the pollution problem.
To address the issue of chromium-contaminated wastewater, various chromium removal techniques have been developed. These include biological methods, such as using different fungal and bacterial species, and adsorption techniques with natural adsorbents or low-cost agricultural wastes. For example, Actinomycetes, Streptomyces rimosus, and Streptomyces griseus have shown promising results in removing chromium from wastewater. Other natural adsorbents, such as clay, zeolites, peat moss, and chitin, have also been effective in removing heavy metals like chromium from industrial wastewater.
In addition, low-cost agricultural wastes like neem leaves, coconut shell, orange peel, banana rachis, and pomegranate husk have been explored for their adsorption potential to remove chromium from tannery effluent. Some innovative methods, such as using pumice (volcanic rock) and rice husk silica powder, have also been investigated, achieving notable reductions in chromium levels.
One simple and cost-effective method to remove chromium from tannery wastewater is by neutralizing the chrome liquor (pH 3.5-4.0) and lime liquor (pH 11-13) produced during the tanning process. Mixing these liquors results in the precipitation of chromium, which can then be removed through filtration. This process significantly reduces the chromium content in the wastewater.
While these methods offer promising solutions for treating tannery effluent and removing harmful chromium species, it is important to note that the tanning industry still faces challenges in implementing these technologies due to high economic and environmental costs.
Coal Plants: Sulfur Dioxide Air Pollution Effects
You may want to see also
Frequently asked questions
Lime is used in the tanning process to treat animal skins and hides and turn them into leather. During this process, at least 300 kg of chemicals, including lime, are added per ton of hides. The use of lime creates a lot of sludge and total solids, which are washed away as wastewater. This wastewater is highly polluted and harmful to the ecosystem, causing the widespread death of living organisms.
Lime in tannery wastewater increases the pH level to 11-13, which is highly alkaline. This high alkalinity can cause chemical burns to the skin, eyes, nose, throat, and lungs. It also poses a variety of health risks to living beings in the area, including damage to liver and kidney function.
Chromium is used in the tanning process and, when mixed with lime, creates highly toxic chromium species. If this mixture is directly discharged into the water ecosystem, it causes severe environmental pollution and poses serious health threats to humans, animals, and plants.
To reduce lime pollution, the tanning industry can adopt cleaner tanning methodologies, such as the use of clean technology in the unhairing process and recycling of unhairing lime-sulfide liquors. There is also a need for technological developments in the avoidance and control of pollution, such as the use of brown seaweed to remove chromium from wastewater.



















