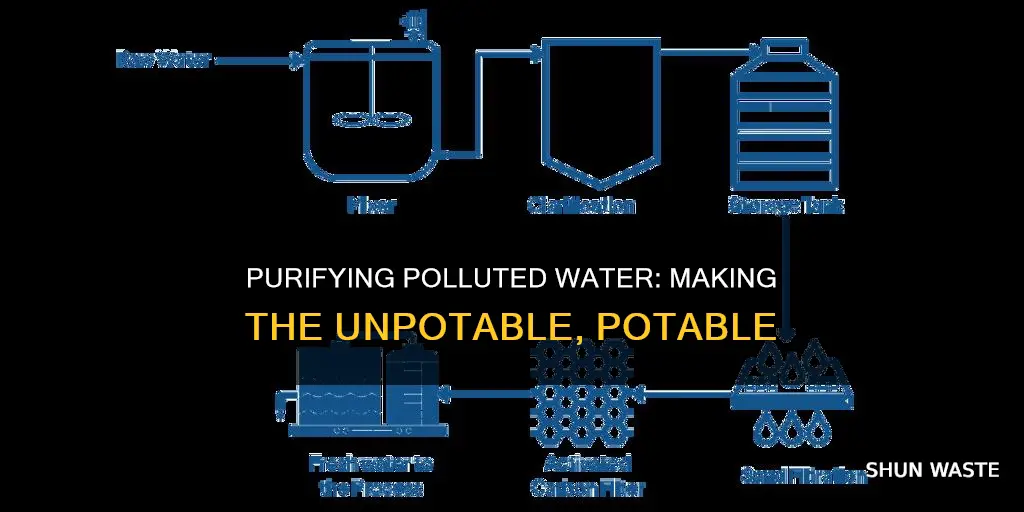
Water is essential for human life, but unfortunately, most water sources have become polluted over time. While tap water is usually potable, it may still contain toxins or debris, and water from natural sources such as rivers is often contaminated with harmful bacteria, viruses, and parasites. To make polluted water potable, it must be purified through processes such as filtration, boiling, chemical disinfection, or ultraviolet light purification. These methods can remove large particles and kill harmful microorganisms, making the water safe for human consumption.
Ways to Make Polluted Water Potable
| Characteristics | Values |
|---|---|
| Boiling | Boil water for at least 60 seconds if at an elevation below 6,500 feet; 3 minutes if above |
| Filtering | Use a filter with an absolute pore size of less than or equal to 1 micron |
| Disinfecting | Use chlorine, iodine, or chlorine dioxide tablets or liquid drops |
| Distilling | Gather water droplets from evaporated steam |
| UV Light Purification | Use a pen-shaped UV light built into a water bottle |
| Reverse Osmosis | Force water through a semi-permeable membrane to remove impurities |

Boiling
However, boiling is not an effective way to remove dissolved chemicals or particulates from water. If you boil brown water, for example, it will still be brown. If the substance causing the discolouration is a toxin, drinking the boiled water is not advisable. Boiling water will also not remove plastic microfibres.
Understanding Pollutants' Pathways into Surface Water
You may want to see also

Using a water filter
Types of Water Filters
There are various types of water filters available, including whole house water filters, portable water filters, and smaller models that attach directly to your sink or fridge via the waterline. When choosing a water filter, consider its effectiveness against bacteria, viruses, and sediments, as well as its ability to remove unpleasant smells and minerals. One popular option is activated charcoal filters, which can extract up to 81 compounds, including herbicides, from water.
Maintaining Your Water Filter
To ensure the longevity of your water filter, it is important to replace the inner filter regularly. For larger models, the inner filter should be changed every six months, while smaller water filters may require replacements more frequently. Additionally, follow the manufacturer's instructions for care and usage to maintain the effectiveness of your water filter over time.
Collecting Water for Filtration
When collecting water for filtration, it is important to prioritise safety. Start with a clean container that has preferably been disinfected prior to use. Wash your hands with soap and water or use hand sanitiser before collecting water to avoid contamination. Choose a collection spot that is at a higher elevation or near the water's source, away from established campsites and animal grazing areas. Collect water from areas of moving water, such as rivers and streams, rather than stagnant water, which can be breeding grounds for insects, bacteria, and viruses.
The Filtration Process
Once you have collected the water, you can begin the filtration process. If your water appears cloudy or contains floating material, it is advisable to filter it even if you plan to boil or disinfect it afterward. Most water filters have a screen with tiny holes that can remove protozoa and some bacteria but may not be effective against viruses due to their small size. To enhance the effectiveness of your water filter, consider using a filtration system with an absolute pore size of less than or equal to 1 micron, which has been proven to be highly effective in removing cryptosporidium and giardia.
Combining Filtration with Other Methods
While filtration is a crucial step in purifying water, it is typically not sufficient on its own to make water potable. It should be followed by boiling or disinfection to kill any remaining bacteria, viruses, or parasites. Boiling is an effective method to kill disease-causing organisms, and the duration of boiling depends on your elevation. Below 6,500 feet, boiling water for 1 minute is sufficient, while at elevations above 6,500 feet, 3 minutes of boiling is recommended. Disinfection can also be achieved through chemical treatment or UV light, which can kill bacteria, viruses, and other harmful organisms.
Precautions and Best Practices
Always follow the manufacturer's instructions for using and maintaining your water filter. Additionally, be mindful of any water quality alerts, such as harmful algal blooms or chemical spills, that may affect the safety of your water source. It is crucial to never drink water from a natural source that has not been properly purified, even if it appears clean. By combining filtration with other purification methods and adhering to safety guidelines, you can effectively make polluted water potable and safe for consumption.
Carbon Pollution Control: Strategies for a Sustainable Future
You may want to see also

Distillation
The distillation process involves three steps: boiling, condensation, and collection. First, contaminated water is heated to a temperature above 212°F (100°C), causing the water to evaporate and leaving behind the majority of contaminants. The steam then rises and enters a condensing chamber, where it is cooled and converted back into liquid water. Finally, the distilled water is collected in a storage container.
However, distillation is less effective at removing certain organic compounds, specifically volatile and semi-volatile organic compounds (VOCs) with boiling points near or lower than that of water. These compounds, such as benzene and toluene, can vaporize along with the water and recontaminate the purified product if not removed prior to condensation. To address this issue, some distillation units include columns or volatile gas vents to eliminate organic chemicals with low boiling points. Additionally, a carbon filter can be used after distillation to trap any lingering contaminants.
Pollution's Impact on Animals: A Toxic Threat
You may want to see also

Chemical disinfection
The choice of disinfectant depends on a variety of factors, including its efficacy against waterborne pathogens (bacteria, viruses, protozoa, and helminths); the accuracy with which the process can be monitored and controlled; its ability to produce a residual that provides an added measure of protection against possible post-treatment contamination; the aesthetic quality of the treated water; and the availability of the technology for the adoption of the method on the scale that is required for public water supplies.
Chlorine
Chlorine is a strong oxidising disinfectant that has been used to treat drinking water supplies for more than 60 years. It is the most widely used method for disinfecting water supplies in the United States. Chlorine has an atomic number of 17, a melting point of -102°C, a boiling point of -35°C, and an oxidation potential of -1.36 V at 25°C. When chlorine is added to water, the following chemical reactions occur:
> Extremely little molecular chlorine (Cl2) is present at pH values greater than pH 3.0 and total chlorine concentrations of less than ~1,000 mg/liter.
> The hypochlorous acid (HOCl) that is produced further ionizes to form hypochlorite ion (OCl-) and hydrogen ion (H+) (Reaction 2).
> The dissociation of hypochlorous acid is dependent chiefly upon pH and, to a much lesser extent, temperature, with almost 100% hypochlorous acid present at pH 5 and almost 100% hypochlorite ion present at pH 10.
> Free available chlorine refers to the concentration of hypochlorous acid and hypochlorite ion, as well as any molecular chlorine existing in a chlorinated water.
Chlorine can be added to water in the form of sodium hypochlorite (NaOCl). Chlorine is a strong oxidising agent and will react with any ammonia (NH3) in the water to form inorganic chloramines. Although inorganic chloramines are less effective oxidising and disinfecting agents than hypochlorous acid and hypochlorite ion, they are more stable and will produce a residual in water that will persist for longer.
Iodine
Iodine is the only common halogen that is a solid at room temperature, and it possesses the highest atomic weight. Of the four common halogens, it is the least soluble in water, has the lowest standard oxidation potential for reduction to halide, and reacts least readily with organic compounds. Iodine reacts with water to form hypoiodous acid (HOI) and iodide ion (I-). The effect of pH on this reaction is shown in Table II-8.
> The distribution of chemical species of iodine given in Table II-8 was taken from the calculations that were made by Chang (1958) from the equilibrium expression:
> The value of Kh, the hydrolysis constant, is given by Wyss and Strandskov (1945) as 3 × 10-12 at 25°C.
> With iodine residuals at 0.5 mg/liter, which are expected in water systems, and a pH of 5, approximately 99% of the total iodine residual is present as iodine and only 1% as hypoiodous acid. At pH 7, the two forms are present in almost equal concentrations. At pH 8, only 12% is present as elemental iodine and 88% as hypoiodous acid, which can be converted to hypoiodite ion (OI-).
Iodine can be added to a municipal water supply by several procedures, including by using nonhazardous solvents and solubilising agents such as ethyl alcohol (C2H5OH) and potassium iodide (KI) to overcome the low concentration of aqueous iodine stock for solution feeders. Another method produces the required concentration of iodine by passing water through a bed of crystalline iodine (saturator).
Chlorine Dioxide
Chlorine dioxide (ClO2) is a powerful oxidising agent that reacts rapidly with most organic and many inorganic compounds. It does not convert chloride to chlorine under test conditions and does not react extensively with ammonia (NH3). It is one of the few stable nonmetallic inorganic free radicals and has more than 2.5 times the oxidising capacity of chlorine. It can be prepared from chlorine and sodium chlorite (NaClO2) through the following reactions:
> Chlorine dioxide may also be prepared from chlorine and sodium chlorite through the following reactions:
> Chlorine dioxide can also be prepared by the addition of a strong acid, such as sulphuric acid (H2SO4) or hydrochloric acid, to sodium chlorite as shown in the following reactions:
Potassium Permanganate
Potassium permanganate (KMnO4) is a strong oxidising agent that has been used as a municipal water treatment chemical since 1913. It is a strong oxidising agent that will react with reducing agents in solutions. It will also oxidise ammonia (NH3). The rates for this reaction increase with pH. Potassium permanganate can be added to water in the form of crystalline potassium permanganate, which is highly soluble in water.
Other Chemical Disinfectants
Other chemical disinfectants include:
- Bromine: a powerful oxidising agent that has been applied to water in the form of liquid bromine (Br2), bromine chloride gas (BrCl), or from a solid brominated ion exchange resin.
- Ferrates: salts of ferric acid (H2FeO4) that are strong oxidising agents.
- Hydrogen peroxide (H2O2): a strong oxidising agent that has been used for disinfection for over a century.
- Silver: a metal that has been known for millennia and has been used as a water disinfectant since the Persian king Cyrus.
- Ultraviolet radiation: electromagnetic or particulate ionising radiation that may be gamma or X-rays, and the particles may be alpha or beta or neutrons, mesons, positrons, or neutrinos.
Human Waste: A Surprising Source of Pollution?
You may want to see also

Reverse osmosis
Osmosis is a natural process in which water molecules pass through a semi-permeable membrane from a region of lower solute concentration to one of higher concentration. This movement is driven by osmotic pressure, which strengthens as the concentration difference grows. The water molecules continue to migrate until the concentrations on both sides of the membrane are equal, establishing osmotic balance. Reverse osmosis, on the other hand, is the opposite process. Pressure is applied to the feed side of the water, and when the pressure exceeds the osmotic pressure of the solution on that side, water flows in the opposite direction of natural osmosis, moving from the region of higher solute concentration to the region of lower solute concentration.
High pressure is applied to the water at the core stage of reverse osmosis, pushing it through the semi-permeable membrane. This membrane, the critical component of the system, features tiny pores (about 0.0001 microns) that allow only water molecules to pass while effectively blocking and removing a significant amount of dissolved salts, chemicals, and microorganisms. The purified water, termed the permeate, is then collected in a storage tank for later use. The concentrated contaminants, which do not pass through the semi-permeable membrane, are expelled as wastewater or "brine." This step involves a flow restrictor and drain system that manages water flow through the membrane, maintaining efficiency and preventing the membrane from becoming overwhelmed by pressure.
The application of reverse osmosis in water treatment is virtually unlimited, given it can be deployed as a complementary technology to other water treatment processes for high-grade purified water. Reverse osmosis is largely revered as a desalination technology due to its high salt rejection rates and tolerance to high TDS levels. Typical reverse osmosis plants lessen the TDS concentrations of seawater from roughly 35,000ppm to 1000ppm or less, which is the universal threshold for freshwater. Reverse osmosis water is pure water produced after a series of pretreatment processes and reverse osmosis processes. As an ideal high-quality drinking water and industrial water, it contains almost no dissolved solids, including salts, metal ions, organic compounds, and so on. The pH of RO water falls within the range of 6.5-6.8, showing weak acidity. The average total dissolved solids (TDS) of reverse osmosis water is 300-500ppm, complying with the drinking water guidelines of the World Health Organization.
Plastic Pollution's Impact on Global Warming: A Complex Link
You may want to see also
Frequently asked questions
Potable water is clean water that is safe to consume and use for other purposes such as brushing your teeth, washing your hands, and preparing food.
There are several ways to make water potable, including:
- Boiling
- Using a water filter
- Distilling
- Using a UV light purifier
- Using iodine
- Using chlorine/bleach
- Reverse osmosis
Consuming polluted water can lead to various health issues such as gastrointestinal illnesses, nervous system or reproductive problems, and chronic diseases like cancer. It can also cause waterborne diseases such as cryptosporidiosis, giardiasis, typhoid fever, cholera, and hepatitis.
Common sources of drinking water contaminants include industry and agriculture, human and animal waste, treatment and distribution processes, and natural sources such as high levels of arsenic, heavy metals, or radionuclides in groundwater.



















