Acetone is a colourless, highly volatile liquid with a sweetish odour. It is used as a solvent in many household products such as nail polish remover, paint thinner, detergents, and cleansers. Acetone is also used in the manufacture of plastics, fibres, and drugs. While it is produced in small quantities in the human body, it is also commonly found in the environment due to industrial processes. Acetone is highly flammable and poses a serious fire hazard. It has a flashpoint of -18°C to -20°C and can cause an explosion if handled improperly. Exposure to acetone can irritate the eyes and respiratory system and lead to various negative health effects.
| Characteristics | Values |
|---|---|
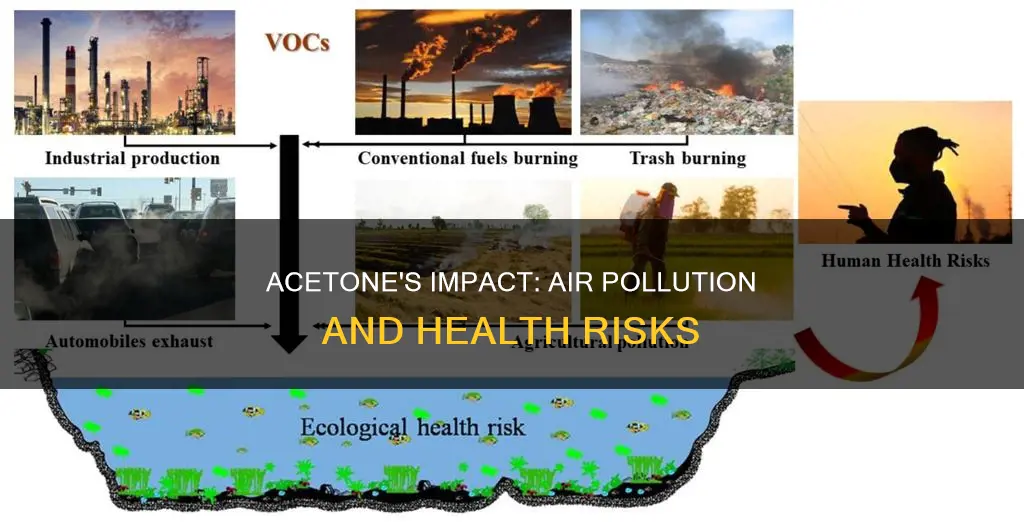
| How acetone enters the air | Acetone is emitted mainly into the air. It can move from the atmosphere into water and soil through rain and snow, and it can quickly evaporate back into the air. Acetone can also enter the air through evaporation from contaminated well water used for household purposes, and through soil vapour intrusion. |
| Acetone sources | Acetone is produced both naturally and through industrial processes. Natural sources include plants, trees, volcanic gases, forest fires, and the breakdown of body fat. Industrial sources include the manufacturing of basic chemicals, plastic products, metals, vehicles, vehicle parts, paints, medicines, and more. |
| Environmental effects | Acetone has a slight toxicity when exposed to aquatic life. It has caused membrane damage, a decrease in size, and a decrease in germination of various agricultural and ornamental plants. |
| Health risks | Exposure to acetone can irritate the eyes and respiratory system and lead to headaches, light-headedness, confusion, increased pulse rate, nausea, vomiting, drowsiness, unconsciousness, and possibly coma. Long-term exposure to high levels of acetone may increase the risk of nervous system toxicity. |
What You'll Learn
- Acetone is emitted into the air through industrial processes and the use of household products
- It is highly flammable and poses a serious fire hazard
- It can cause irritation to the eyes and respiratory tract, as well as nervous system effects such as headaches and dizziness
- It has been linked to kidney, liver, and nerve damage in animals
- It is not considered a carcinogen or a mutagen but can cause skin and eye irritation

Acetone is emitted into the air through industrial processes and the use of household products
Acetone is a colourless, highly volatile liquid with a pungent, sweet odour. It is used in a wide range of industrial processes and is also present in many household products. Due to its widespread use, acetone is emitted into the air through various activities, contributing to air pollution.
Acetone is commonly used as an industrial solvent for fats, oils, waxes, resins, rubber, plastics, lacquers, varnishes, and rubber cements. It is also used in the manufacturing of chemical compounds, rayon, photographic films, plastics, fibres, drugs, and other chemicals. Additionally, acetone is used for storing acetylene gas, purifying paraffin, and hardening and dehydrating tissues. These industrial applications often release acetone into the air, contributing to air pollution.
In addition to industrial sources, acetone is also emitted into the air through the use of household products. It is a common ingredient in domestic products such as aerosol paints, architectural coatings, automotive and machinery paints, furniture polish, cleaners, laundry pre-soaks, pet flea treatments, and nail polish and polish remover. The use and evaporation of these products can release acetone vapours into the air, contributing to indoor and outdoor air pollution.
Moreover, acetone can enter the indoor air of homes through evaporation from contaminated well water used for household purposes. It can also migrate into buildings through soil vapour intrusion, where it evaporates from groundwater, enters the soil vapour, and moves through building foundations. While outdoor air typically contains lower concentrations of acetone, it can still be a source of the chemical indoors.
To minimise the impact of acetone pollution, it is important to take measures to limit exposure and reduce indoor air levels. Removing household sources of acetone, improving ventilation, and using activated carbon filters on water supplies can help mitigate the presence of acetone in indoor air.
Irrigating with Polluted Water: Safe for Oxygen Not Included?
You may want to see also

It is highly flammable and poses a serious fire hazard
Acetone is a highly volatile, flammable liquid with a pungent odour. It has a flashpoint of 0°F (-18°C) and is classified as a Class 1B flammable liquid by the NFPA 30. This means that it requires specialised storage to mitigate the risk of fire. Acetone has a flame temperature of 1980°C and burns with a dull blue flame in small amounts. In larger amounts, fuel evaporation causes incomplete combustion, resulting in a bright yellow flame.
Air mixtures containing 2.5-12.8% acetone by volume may explode or cause flash fires when hotter than the liquid's flashpoint of −20°C (−4°F). Acetone vapours can travel along surfaces and flash back to distant ignition sources. Although acetone has a high ignition initiation energy, and accidental ignition is rare, static discharge can still ignite its vapours.
The safe storage of acetone is critical due to its highly flammable nature. Drums or totes containing bulk quantities of acetone should be stored in customised flammable liquid storage drum lockers. These lockers are designed to provide either 2-hour or 4-hour fire-rated storage, depending on the location relative to occupied buildings. Additionally, these lockers can be configured for easy forklift access and fitted with explosion-proof accessories to comply with local regulatory requirements.
For smaller quantities of acetone, the NFPA 30 provides specific guidelines for storage. Up to 120 gallons can be stored outside an approved safety storage building, while quantities exceeding 120 gallons must be kept inside a designated flammable liquid storage safety building. No more than 60 gallons can be stored in a single building or locker. When storing more than 240 gallons in a single building, the International Building Code mandates compliance with H-occupancy requirements.
To ensure safety during the decanting process, it is crucial to electrically bond the containers to prevent static discharge from causing a fire. Additionally, when mixing or dispensing acetone, explosion relief panels and an explosion-proof electrical system are necessary. These precautions are essential to mitigate the risk of fire and ensure the safe handling of acetone.
Filtering Ground-Level Pollution: Innovative Ways to Breathe Easier
You may want to see also

It can cause irritation to the eyes and respiratory tract, as well as nervous system effects such as headaches and dizziness
Acetone is a colourless liquid with a sweet odour that can be man-made or produced in small quantities in the human body through the normal breakdown of fat. Exposure to large amounts of acetone in air over short periods of time has been linked to irritation of the eyes and respiratory tract, as well as nervous system effects such as headaches, lightheadedness, dizziness, unsteadiness and confusion.
Acetone is a known eye irritant, with occupational exposure causing irritation to the eyes. In addition, exposure to acetone in the workplace for longer periods has been linked to decreased scores on tests used to evaluate nervous system function.
Acetone exposure has also been associated with irritation of the nose, throat, trachea and lungs. This has been observed in both workers exposed to acetone occupationally and in volunteers under controlled laboratory conditions.
The human and animal data indicate that long-term human exposure to acetone may increase the risk of nervous system toxicity.
Andromeda Viewing: No Sky Pollution, What Can We See?
You may want to see also

It has been linked to kidney, liver, and nerve damage in animals
Acetone is a colourless, highly volatile, and flammable liquid with a pungent odour. It is used as a solvent in many household products, such as nail polish remover, paint thinner, and varnish remover. It is also used in industrial processes and is naturally produced in small quantities in the human body through the breakdown of fat.
While acetone is generally recognised as having low acute and chronic toxicity, exposure to moderate or high levels can have adverse health effects. When acetone is inhaled or ingested, it enters the bloodstream, exposing all internal organs to the substance. At high levels, acetone can irritate the eyes and respiratory tract and cause nervous system effects, including headaches, dizziness, and confusion.
Animal studies have shown that long-term exposure to acetone can lead to kidney, liver, and nerve damage. In animals, acetone has been linked to an increased risk of birth defects and male infertility. However, it is unknown if these effects would occur in humans exposed to similar levels.
To minimise the potential risks associated with acetone exposure, it is important to follow safety guidelines when using products containing acetone. Proper ventilation and avoiding use near open flames are crucial due to acetone's high flammability. Additionally, washing the skin with water can help prevent skin irritation or dryness.
Pollution's Surprising Benefits: Boon or Bane for Nature?
You may want to see also

It is not considered a carcinogen or a mutagen but can cause skin and eye irritation
Acetone is a colourless, highly volatile liquid with a pungent odour. It is used in a variety of household products, such as nail polish remover, paint thinner, and detergents, and is produced and disposed of in the human body through normal metabolic processes.
While acetone is not considered a carcinogen or mutagen, it can cause skin and eye irritation. It is important to take precautions when using products containing acetone to avoid these issues. For example, when working with acetone, it is recommended to wear chemical protective clothing, such as gloves and goggles, to avoid skin and eye contact.
Inhalation of acetone can also be harmful, particularly at high concentrations, as it can irritate the nose and throat and harm the nervous system. Symptoms of nervous system harm may include headaches, nausea, dizziness, drowsiness, and confusion. Skin contact with acetone may cause mild irritation and, in some cases, dry, red, cracked skin (dermatitis). Ingesting large amounts of acetone can also lead to similar effects as inhalation.
Overall, while acetone is not considered a carcinogen or mutagen, it is important to take the necessary precautions to avoid skin and eye irritation and potential nervous system harm from exposure.
Pollution's Silver Lining: Exploring Unlikely Benefits
You may want to see also
Frequently asked questions
Acetone (2-propanone or dimethyl ketone) is an organic compound with the formula (CH3)2CO. It is a colorless, highly volatile, and flammable liquid with a pungent sweet odor.
Acetone is used as a solvent for fats, oils, waxes, resins, rubber, plastics, lacquers, varnishes, and rubber cements. It is also used to make many chemical compounds, including rayon, photographic films, plastics, fibers, and drugs.
Acetone is emitted mainly into the air. It can move from the atmosphere into water and soil through rain and snow and quickly evaporate back into the air. Acetone can also enter homes through soil vapor intrusion, where it evaporates from groundwater, enters soil vapor, and migrates through building foundations into indoor air.
Exposure to high levels of acetone in the air can irritate the eyes and respiratory tract and cause nervous system effects such as headaches, lightheadedness, dizziness, unsteadiness, and confusion. Long-term exposure may increase the risk of nervous system toxicity.
Acetone has a slight toxicity when exposed to aquatic life, causing membrane damage and decreased size and germination in various agricultural and ornamental plants. It does not bioaccumulate in plants, animals, or humans. In the atmosphere, acetone has a 22-day half-life and is degraded by UV light via photolysis.



















