The pH of water is a crucial indicator of water quality and is measured on a scale of 0 to 14, with 7 being neutral. pH levels below 7 indicate acidity, while levels above 7 are basic. Pollution can significantly impact the pH of water, and subsequently, harm plants and animals in the water. This is because pH determines the solubility and biological availability of chemical constituents such as nutrients and heavy metals. For instance, pollutants from coal mining can drastically lower the pH of water, making it highly acidic and toxic. Additionally, increased atmospheric carbon dioxide (CO2) levels can lower the pH of water, as CO2 is soluble in water, forming weak carbonic acid.
| Characteristics | Values |
|---|---|
| pH level of uncontaminated water | 7.0 (neutral) |
| pH level of contaminated water | Less than 7 (acidic) |
| Effect of low pH on water taste | Bitter |
| Effect of low pH on water pipes and appliances | Encrusted with deposits |
| Effect of low pH on chlorine treatment | Reduced effectiveness |
| Effect of low pH on metals | Increased solubility |
| Effect of low pH on plants | Increased absorption of heavy metals |
| Effect of low pH on aquatic life | Harmful |
| Effect of low pH on soil | Changes availability of chemical elements |
| Effect of high pH on heavy metals | Reduced toxicity |
| Effect of high pH on water pipes and appliances | Build-up of calcium and magnesium carbonate |

Acidic deposition
Acid deposition is a significant environmental concern, altering the pH of water and causing far-reaching ecological damage. It is primarily caused by the release of certain gases and particles into the atmosphere, which are then transported and deposited onto the Earth's surface. This process, known as acid deposition, includes both dry and wet deposition.
Dry deposition occurs when acidic gases and particles are directly released into the atmosphere and settle onto surfaces without first being dissolved in an aqueous medium like rain or clouds. This can account for a substantial proportion of total acid deposition, with particles and gases adhering to the ground, plants, and other surfaces.
Wet deposition, on the other hand, involves the transport and deposition of acids onto surfaces after they have been dissolved in precipitation, such as rain, snow, or clouds. This process is commonly referred to as acid rain, and it occurs when compounds in the atmosphere, such as sulfur dioxide and nitrogen oxides, react with water to form sulfuric acid and nitric acid, respectively. Acid rain has a pH of around 4, making it harmful to both the environment and humans.
The principal sources of the compounds that cause acid rain are human activities such as electricity generation, animal agriculture, factories, motor vehicles, and power plants. The burning of sulfur-containing coal for heat and electricity is a significant contributor, particularly in China, Russia, and areas downwind of them. The use of tall smokestacks to reduce local pollution has inadvertently contributed to the spread of acid rain by releasing gases into regional atmospheric circulation.
The effects of acid deposition are wide-ranging and detrimental. It can alter soil composition, water pH, and nutrient content in affected areas. Soils compromised by acid deposition lose their ability to neutralize strong acids, impacting plant growth and ecosystem recovery. Acid deposition also affects water bodies, changing their pH levels and, consequently, the solubility and availability of nutrients and heavy metals. This can have harmful effects on aquatic organisms and plants living in the water.
To address the issue of acid deposition, the US Congress passed the Acid Deposition Act in 1980, establishing the National Acidic Precipitation Assessment Program (NAPAP). This program expanded the monitoring of acidic precipitation, aiming to determine long-term trends and assess the impact of acid rain on ecosystems, historical structures, and human health.
Dams: Water Pollution or Conservation?
You may want to see also

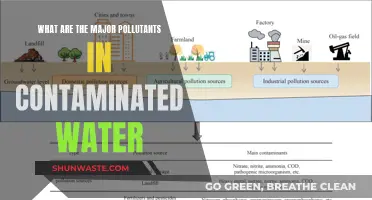
Industrial pollutants
Sources of Industrial Pollutants
Industrial sources of acidic gases, such as power plants and pulp mills, emit sulfur and nitrogen oxides into the atmosphere. These gases are then transformed into H2SO4 and HNO3, which are deposited into freshwater systems as acidic deposition. This process increases the levels of SO42-, NO3-, and H+ in the water, leading to a decrease in pH.
Another source of industrial pollution is the coal industry. A case study by Scullion J. and Edwards RW. (1980) investigated the effects of coal industry pollutants on the macroinvertebrate fauna in a small river in the South Wales coalfield. They found that drainage water from coal stockpiles and seasonal flows could cause pH fluctuations in the river.
Effects of Industrial Pollutants on Water pH
The direct dumping of industrial pollutants into water bodies can decrease the pH, making the water more acidic. This is particularly harmful when the water has a low buffering capacity, as it can quickly reach toxic pH levels. Acidic water can corrode metal pipes and increase the solubility of heavy metals, making them more bioavailable and toxic to aquatic life.
Mining activities can also produce acidic runoff by exposing rocks to rainwater, leading to mining drainage that introduces acidic elements into waterways. This can have detrimental effects on the ecosystem, as seen in a study of the Tarkwa mining area, where the quality of water supply systems was highly affected by mine contaminants, mining-related activities, and improper waste disposal.
Impact on Aquatic Life
The change in water pH caused by industrial pollutants can have significant effects on aquatic life. More fragile plants and animals may struggle to survive in very low or high pH conditions. Additionally, microorganisms in the water can be affected, potentially disrupting the entire aquatic food chain.
Furthermore, increased acidity in water can make certain chemicals more toxic. For example, at low pH, chemicals like cyanide and sulfide become more toxic, posing a threat to any organisms living in the water.
Impact on Water Quality
Water with a low pH tends to have an unpleasant metallic taste or odor, making it unsuitable for human consumption. Additionally, highly acidic water can cause corrosion in metal pipes and appliances, leading to further issues.
In summary, industrial pollutants can have far-reaching consequences on water pH, affecting both the ecological balance and the quality of water for human use. It is crucial to address and mitigate these pollutants to maintain the health of aquatic ecosystems and ensure safe drinking water supplies.
Ants and Water: Pollution and Its Impact
You may want to see also

Mine drainage
Pollution can significantly impact the pH of water, and one of the most notable examples of this is mine drainage. Mine drainage, also known as acid mine drainage (AMD) or acid rock drainage (ARD), is a significant environmental issue stemming from the mining industry. It occurs when water comes into contact with exposed rocks, tailings, and overburden, initiating an oxidation process that generates acidity. This process is particularly prevalent in abandoned mines, where pumping has ceased and water has flooded the mine, providing the necessary conditions for oxidation to take place.
The oxidation of certain minerals, such as pyrite (iron sulfide) and other sulfide minerals, in the presence of water and oxygen, leads to the production of acids. This results in the outflow of acidic water, which can have detrimental effects on the surrounding environment, particularly aquatic ecosystems. The acidity of mine drainage is further enhanced by the presence of oxidizing bacteria, known as extremophiles or acidophiles, which thrive in the low pH conditions of abandoned mines.
The impact of mine drainage on water pH and the subsequent release of heavy metals can be mitigated through various treatment methods. Constructed wetlands, for example, have been proposed to treat acid mine drainage by utilizing natural processes such as metal precipitation and the interaction with organic matter. Additionally, limestone-based treatments can be employed to neutralize acidic waters, although their effectiveness depends on factors such as contact time and water flow rate.
The understanding and management of mine drainage are crucial to minimizing its environmental and health impacts. Geochemical assessments can be conducted to predict the likelihood and potential effects of acid mine drainage before selecting a mine site. Additionally, programs like the Mine Environment Neutral Drainage (MEND) program in Canada aim to reduce the effects of acid mine drainage and mitigate its impact on local water resources.
Purifying Polluted Water in Oxygen: Strategies for Success
You may want to see also

Soil composition
Soil pH can be influenced by several factors, including natural processes and human activities. Natural variations in soil pH occur due to differences in geological materials, parent rock, and organic matter content. Human activities, such as agricultural practices, can also significantly alter soil pH over time. For example, the application of fertilizers or lime can change the soil's acidity or alkalinity.
The pH of soil water is directly related to the pH of the soil itself. However, the type and quantity of the solution used for measurement can impact the results. When soil is suspended with water, pH values tend to be higher compared to using a calcium chloride (CaCl2) solution. Laboratory testing is often required to obtain accurate pH values, and the suspension method can vary between laboratories.
Maintaining the optimal soil pH is crucial for agriculture and environmental health. Soil pH affects the availability of nutrients to plants, with most mineral nutrients being readily available when the pH is near neutral. Strongly acidic soils (pH less than 5.5) can hinder plant growth, while extremely alkaline soils (pH greater than 9) are likely to have high sodium levels and nutrient deficiencies. Therefore, regular monitoring of soil pH is important to identify and correct potential nutrient deficiencies, ensuring optimal plant growth and soil health.
Minimizing Water Pollution: Strategies to Reduce Aquatic Contamination
You may want to see also

Heavy metals
Pollution can significantly impact the pH of water, and this change in pH can, in turn, affect the plants and animals living in the water. For instance, water that exits an abandoned coal mine can be extremely acidic with a pH of 2, which would be harmful to any fish attempting to live in it.
The pH of water determines the solubility and biological availability of heavy metals. Heavy metals tend to be more toxic at lower pH levels because they are more soluble. In other words, as the pH of water decreases and becomes more acidic, the solubility of heavy metals increases, making them more available for uptake by aquatic organisms. This increased solubility can result in higher concentrations of heavy metals in the water, posing a greater risk to the health of aquatic life and potentially entering the food chain.
The detection and monitoring of heavy metals in water are influenced by pH. Techniques such as laser-induced breakdown spectroscopy coupled with a phase transformation method (LIBS-PT) have been employed to detect heavy metals in wastewater. The sensitivity of detecting these metals can be enhanced by optimizing the pH of the solution. Additionally, leaching tests have demonstrated a strong dependence of heavy metal leaching on pH, with the highest concentrations observed in acidic environments.
Overall, heavy metal pollution in water systems is a significant concern, and understanding the interplay between pH and heavy metal toxicity is crucial for assessing and mitigating the potential ecological and public health risks associated with contaminated water sources.
Water Pollution in Iowa: Is It a Concern?
You may want to see also
Frequently asked questions
Pollution can cause a decrease in the pH of water, making it more acidic. This can be caused by the dumping of chemicals, industrial pollutants, and acid rain. Acid rain is caused by nitrogen oxides and sulfur dioxide combining with water vapour, which comes from automobile and coal-fired power plant emissions.
A change in pH can affect the taste of water, with low pH water tasting metallic or bitter. It can also affect the effectiveness of chlorine as a disinfectant, with lower pH levels reducing its efficacy. Increased acidity in water can also cause certain minerals to dissolve, releasing metals and other chemical substances.
A change in pH can harm aquatic life by increasing the solubility of heavy metals, making them more available to be taken up by plants and organisms. It can also affect the availability of nutrients, such as phosphorus and nitrogen, which can impact the survival of aquatic organisms.