Nutrient pollution is a process where too many nutrients, primarily nitrogen and phosphorus, are added to bodies of water. This can act as a fertilizer, causing excessive growth of algae. When the algae die, they decay, and this process uses up oxygen in the water, leading to low levels of dissolved oxygen. This can harm aquatic life and affect water quality. Certain human activities, such as agriculture, residential practices, and industrial operations, contribute to nutrient pollution and subsequent oxygen depletion. Eutrophication, or excess nutrients in water bodies, is often listed as a reason for impairment under the Clean Water Act.
| Characteristics | Values |
|---|---|
| Definition of dissolved oxygen | Refers to the level of free oxygen present in water |
| Nutrient pollution | When too many nutrients, mainly nitrogen and phosphorus, are added to bodies of water |
| Impact of nutrient pollution on dissolved oxygen | Nutrient enrichment stimulates oxygen-generating (photosynthesis) and oxygen-depleting (respiration) processes |
| Impact of low dissolved oxygen | Can harm aquatic life and affect water quality |
| Sources of nutrient pollution | Wastewater treatment facilities, runoff from land in urban areas during rains, and farming |
| Eutrophication | Excess nutrients in streams, causing them to be listed as impaired |
| Impact on aquatic life | Low dissolved oxygen can kill fish, crabs, oysters, and other aquatic animals |
| Factors influencing dissolved oxygen | Water temperature, ionic strength, and dissolved solids |
| Impact of temperature | Colder, deeper fresh waters can hold higher concentrations of dissolved oxygen |
What You'll Learn

Eutrophication and organic pollution
Nutrient pollution is a major cause of eutrophication, which can have detrimental effects on aquatic ecosystems and human survival. Eutrophication is a process in which nutrients accumulate in a body of water, leading to excessive plant and algal growth. This growth can deplete the oxygen in the water, creating
Eutrophication can occur naturally over centuries as lakes age and fill with sediments. However, human activities have accelerated eutrophication rates through point-source discharges and non-point loadings of limiting nutrients such as nitrogen and phosphorus into aquatic ecosystems. This is known as cultural eutrophication and can occur in both freshwater and saltwater bodies, with shallow waters being the most susceptible.
Cultural eutrophication is caused by the release of sewage, industrial wastewater, fertilizer runoff, and other nutrient sources into the environment. These nutrients stimulate oxygen-generating photosynthesis and oxygen-depleting respiration processes. The increased plant and algal growth due to eutrophication can limit light penetration, reducing growth and causing die-offs of littoral zone plants. It can also impair the chemosensory abilities of organisms that rely on perceiving dissolved chemical cues for survival.
When the dense algal blooms die, microbial decomposition severely depletes dissolved oxygen, creating a hypoxic or anoxic environment that kills aerobic organisms such as fish and invertebrates. This also affects terrestrial animals by restricting their access to water sources for drinking. Eutrophication can lead to structural and functional disruption of aquatic ecosystems and their food webs, resulting in habitat loss and reduced biodiversity.
To combat cultural eutrophication, sewage treatment plants can be upgraded for better biological nutrient removal, and laws regulating sewage discharge and treatment have proven effective in reducing nutrient pollution. Additionally, introducing bacteria and algae-inhibiting organisms, such as shellfish and seaweed, can help control the growth of cyanobacteria, the main source of harmful algae blooms.
The Truth About Carbon Dioxide: Pollutant or Not?
You may want to see also

Increased water temperatures
Water temperature is a vital parameter in assessing the health of a river water body's ecosystem. As water temperatures increase, the solubility of oxygen decreases, leading to a decline in dissolved oxygen (DO) levels. This phenomenon is due to the inverse relationship between dissolved oxygen and temperature. The rise in water temperature causes the gas and water molecules to gain more energy, breaking the weak molecular interactions between them and allowing oxygen to escape.
The impact of increased water temperatures on DO levels is particularly notable during the summer months and the daytime, when higher sunlight penetration warms the water's surface. Climate change is expected to exacerbate this issue, with projected increases in river water temperatures (RWTs) of up to 7.8°C for certain basins by 2071-2100. Consequently, DO saturation capacity is predicted to decrease by 2-12% over Indian catchments during this period.
The decrease in DO levels due to higher water temperatures can have detrimental effects on aquatic habitats and organisms. When DO levels drop below 3 mg/L, conditions become hypoxic, which is lethal to aquatic plants and animals. Warmer water temperatures also contribute to increased metabolic rates, which further deplete oxygen levels through heightened respiration and nitrification.
Furthermore, increased water temperatures can indirectly influence DO levels by interacting with other stressors. For example, warm effluents may contribute to decreased DO concentrations. Additionally, higher temperatures can enhance the growth of harmful algal blooms (HABs), which can deplete oxygen levels during their decay. These complex interactions between temperature, oxygen solubility, and ecological processes underscore the urgency of addressing climate change and mitigating its impact on water temperatures to ensure the health and sustainability of aquatic ecosystems.
Exploring the Next Town Over: Unveiling Local Treasures
You may want to see also

Nutrient sources
Nutrient pollution is primarily caused by an excess of nutrients, usually nitrogen and phosphorus, entering bodies of water. This can act as a fertilizer, causing excessive growth of algae, which is known as eutrophication. This process can lead to low levels of dissolved oxygen in the water as the algae and seagrass die and decay, depleting the oxygen in the water.
There are two main types of nutrient pollution sources: point sources and non-point sources. Point sources are direct pathways from the source to the water, such as sewage pipes, septic tanks, and stormwater drains. These are relatively easy to regulate and manage. Non-point sources, on the other hand, are more challenging to control as they come from diffuse and ill-defined sources. Examples include runoff from agricultural fields, urban areas, and natural landscapes.
Agricultural practices are a significant contributor to nutrient pollution. The use of chemical fertilizers and manure in crop production can result in excess nitrogen and phosphorus entering water bodies. When crops cannot fully utilize all the applied nutrients, the surplus can run off into nearby water sources, impacting water quality. Additionally, agricultural animal production, especially concentrated animal feeding operations (CAFO), release large amounts of animal waste, which contains high levels of nutrients.
Urban and suburban areas also contribute to nutrient pollution. Stormwater runoff from roads, parking lots, and rooftops can carry pollutants, including nutrients from fertilizers and pet waste, into local waterways. Excessive fertilizer use on lawns and gardens can further exacerbate the problem. Municipal sewage treatment plants and motor vehicle emissions are other sources of nutrient pollution in urban settings.
Industrial activities play a role as well. Wastewater discharges from various industries and air pollution emissions, such as those from electric power plants, can introduce nutrients into water and the atmosphere. The burning of fossil fuels, particularly in electric power generation, industry, transportation, and agriculture, has increased the levels of nitrogen in the air and contributed to nutrient pollution.
How Boating Impacts the Environment and Causes Pollution
You may want to see also

Algal blooms
When the concentrations of nitrogen and phosphorus increase in a water body, the right combination of temperature, sunlight, and low flow can trigger an algal bloom. This proliferation causes blooms of algae that turn the water noticeably green, although other colours can occur. Some species of algae grow in clumps covered in a gelatinous coating, allowing them to float and stick together into large surface scums in calm weather. Other algae form thick mats that float on or just below the surface along the shoreline.
The growth of algae consumes oxygen and blocks sunlight from underwater plants. When the algae eventually die, the oxygen in the water is further consumed by bacteria, making it impossible for aquatic life to survive. This results in the formation of "'dead zones" where most life cannot be supported due to depleted oxygen levels.
Harmful algal blooms (HABs) are a growing global concern as they pose risks to human and aquatic ecosystem health and cause economic damage. Certain species of algae, such as cyanobacteria, produce toxins called cyanotoxins that can cause acute and chronic illnesses in humans, pets, and aquatic life. These blooms can also contaminate drinking water sources for nearby communities, causing further health issues.
N95 Masks: Effective Shields Against Pollution?
You may want to see also

Aquatic ecosystem health
Aquatic ecosystems are highly sensitive to nutrient pollution, which can have detrimental effects on water quality and the health of aquatic organisms. Nutrient pollution occurs when excessive nutrients, primarily nitrogen and phosphorus, are introduced into water bodies. These nutrients act as fertilisers, leading to excessive growth and blooms of algae. While algae itself is not inherently harmful, the process of microbial decomposition by bacteria and fungi consumes oxygen, depleting the dissolved oxygen levels in the water.
Dissolved oxygen (DO) is essential for the survival and growth of many aquatic organisms, including fish, crabs, oysters, and other aquatic animals. It is also utilised by microbes for decomposing organic material at the bottom of water bodies, contributing to nutrient recycling. However, when there is an excess of decaying organic matter, such as dying algae, the oxygen at lower water levels is rapidly depleted. This depletion of DO can have severe consequences for aquatic life, as aerobic aquatic organisms require oxygen for their survival.
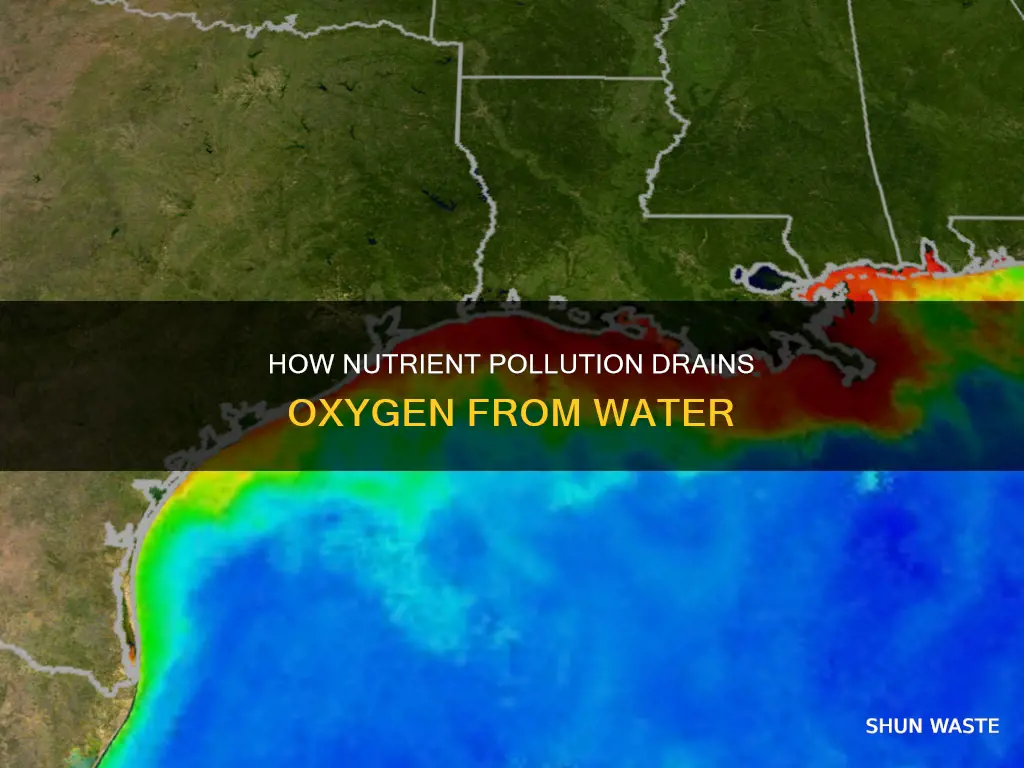
Nutrient pollution-induced oxygen depletion, or eutrophication, has been observed in various water bodies, including the Gulf of America "dead zone" south of Louisiana. This seasonal occurrence is attributed to the nutrient-rich discharge from the Mississippi and Atchafalaya Rivers, leading to algal blooms and subsequent oxygen depletion in subsurface waters. Eutrophication is often listed as a reason for streams being classified as impaired under the Clean Water Act.
The health of aquatic ecosystems is closely tied to maintaining appropriate levels of DO. While colder, deeper fresh waters can hold higher concentrations of DO, various factors can contribute to decreased DO levels. These factors include increased water temperatures, higher ionic strength, increased sediment, and slower water velocities. Human activities, such as agricultural, residential, and industrial practices, can introduce chemical contaminants and organic matter, further contributing to DO depletion.
To preserve the health of aquatic ecosystems, it is crucial to address nutrient pollution and its impact on dissolved oxygen levels. This involves implementing effective management strategies to reduce nutrient inputs, particularly nitrogen and phosphorus, into water bodies. By mitigating nutrient pollution, we can help restore the delicate balance of aquatic ecosystems and ensure the survival and thriving of diverse aquatic organisms.
Exploring the Meaning of Pemberley's Pollution
You may want to see also
Frequently asked questions
Nutrient pollution is the process where too many nutrients, mainly nitrogen and phosphorus, are added to bodies of water. These nutrients act like fertilizers, causing excessive growth of algae.
Nutrient enrichment stimulates oxygen-depleting processes, such as respiration. In addition, the oxygen in the water is used up during the decay of algae and seagrass, leading to low levels of dissolved oxygen.
Low dissolved oxygen levels can harm aquatic life and affect water quality. It can kill fish, crabs, oysters, and other aquatic animals.
Certain human activities, such as agricultural, residential, and industrial practices, contribute to nutrient pollution. These practices introduce chemical contaminants, organic loading, and nutrients to water bodies through various sources, including wastewater treatment plants, fertilizers, and animal wastes.







