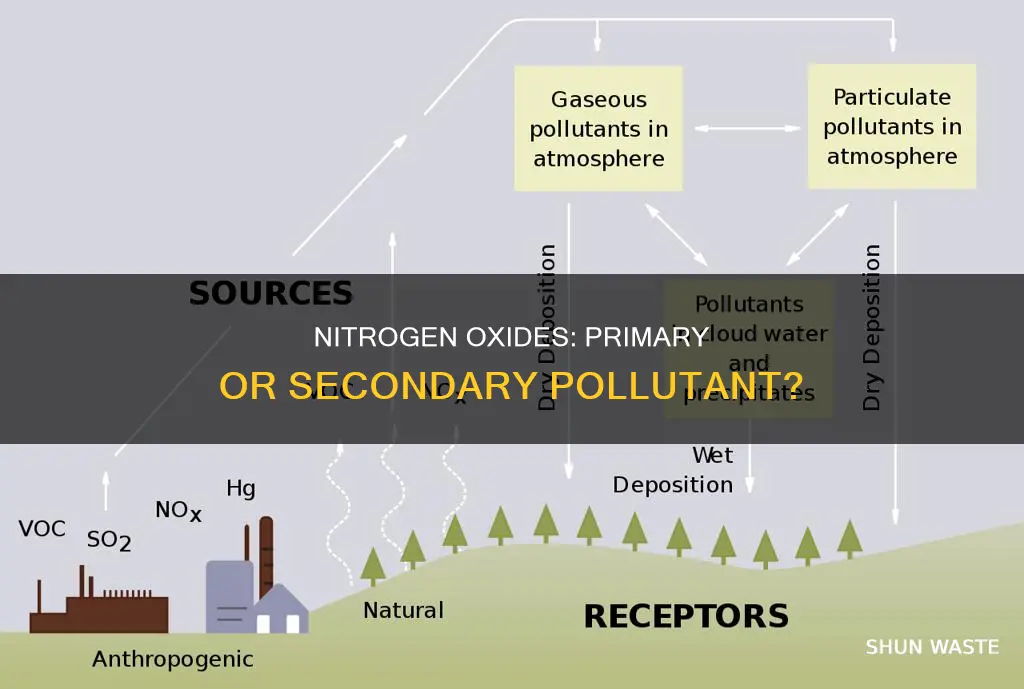
Nitrogen oxides (NOx) are a group of highly reactive gases, including nitrogen dioxide (NO2), nitric oxide (NO), nitrous oxide, nitrous acid, and nitric acid. They are primarily produced through the combustion of fossil fuels like coal, oil, and gas, as well as from vehicle emissions, power plants, and industrial activities. NOx gases can be both primary and secondary pollutants, depending on their formation. When emitted directly into the environment from combustion processes, they are considered primary pollutants. However, NOx can also form in the atmosphere when other chemicals react, making them secondary pollutants.
| Characteristics | Values |
|---|---|
| Type of Pollutant | Secondary Pollutant |
| Composition | One nitrogen atom and two oxygen atoms (NO2) |
| Odor | Foul-smelling |
| Sources | Vehicle emissions, power plants, off-road equipment emissions, burning fossil fuels |
| Reactants | Volatile organic compounds (VOCs), other chemicals in the air |
| Products | Ozone, particulate matter, acid rain, toxic chemicals |
| Health Effects | Harmful when inhaled, affects respiratory system |
| National Ambient Air Quality Standards (NAAQS) | 100 ppb averaged over one hour, 53 ppb averaged over one year |
What You'll Learn

Nitrogen dioxide is a primary pollutant from fossil fuel burning
Nitrogen dioxide (NO2) is a primary pollutant that is emitted as a result of fossil fuel combustion. It is a reddish-brown, highly reactive gas with a foul smell. It is primarily produced in fuel combustion processes from nitrogen and oxygen in the atmosphere. NO2 is formed when fuels burn at high temperatures in vehicles, power plants, industrial processes, and equipment involving fuel combustion.
NO2 is a significant component of air pollution, which is responsible for millions of premature deaths globally. It is harmful to human health, causing respiratory issues and contributing to cardiovascular diseases. Long-term exposure to NO2 has also been linked to adverse perinatal outcomes and lung cancer.
The combustion of fossil fuels, such as coal, oil, and gas, is the primary source of NO2 emissions. This includes high-temperature combustion in transportation, industry, and power generation. Household sources of nitrogen oxides include furnaces, fireplaces, and gas stoves and ovens. These indoor combustion processes can emit harmful pollutants, such as NO2, contributing to indoor air pollution.
NO2 also reacts with other chemicals in the air to form secondary pollutants. These reactions with volatile organic compounds (VOCs) and other gases lead to the formation of particulate matter, ozone, acid rain, and other toxic chemicals. These secondary pollutants further exacerbate the health and environmental impacts of NO2 emissions.
While non-fossil fuel sources, such as biomass burning and microbial N cycles in soils, also contribute to nitrogen oxide emissions, fossil fuel combustion has been the dominant factor since the Industrial Revolution. Regional human activities influence the spatial and temporal patterns of NOx emissions, with non-fossil fuel sources accounting for a significant proportion of total emissions in certain regions.
Red Alert: Is the US Prepared?
You may want to see also

NOx is a secondary pollutant formed from other chemicals
Nitrogen oxides (NOx) are a group of highly reactive gases, including nitric oxide (NO) and nitrogen dioxide (NO2). NOx is considered a secondary pollutant as it is formed through the reaction of NO and NO2 with other chemicals in the air.
NO is the primary pollutant that is directly emitted into the atmosphere. It is produced primarily through fuel combustion processes, such as burning fossil fuels like coal, oil, and gas. This includes vehicle emissions, power plants, and other industrial and domestic combustion sources. NO is then converted to NO2 either within the exhaust system of the combustion device or in the atmosphere through oxidation by ozone.
NO2 is both a primary and secondary pollutant. While it is directly emitted in small proportions, it is also formed through the oxidation of NO. NO2 has a reddish-brown colour and a pungent, acrid odour. It is highly reactive and combines with volatile organic compounds (VOCs) in the presence of sunlight and heat to create ozone (O3).
Ozone is a major secondary pollutant formed from NOx. It is produced through the reaction of NO2 with VOCs, as well as the combination of released oxygen molecules with oxygen (O2) in the atmosphere. Other secondary pollutants formed from NOx include particulate matter, acid rain, and other toxic chemicals. These pollutants have harmful effects on human health, particularly for children, seniors, and individuals with existing lung and heart conditions.
The formation of secondary pollutants from NOx is a complex process influenced by various factors. The presence of sunlight and heat, for example, facilitates the creation of ozone from NO2 and VOCs. Additionally, the concentration of NOx in the atmosphere is influenced by emissions from traffic and urban sources, with higher concentrations observed in large conurbations and areas with heavy traffic.
Pollution Tax: Are Companies Paying Their Fair Share?
You may want to see also

NOx is emitted from vehicles, machinery, and power plants
Nitrogen oxides (NOx) are a group of highly reactive gases that are harmful to both human health and the environment. NOx is primarily produced through the combustion of fossil fuels, such as coal, oil, and gas, and is emitted from vehicles, machinery, and power plants.
Vehicles, including cars, trucks, and other machinery that burn fuel, are a significant source of NOx emissions. These emissions occur due to the high temperatures in the engine cylinders during fuel combustion. While diesel fuel does not contain nitrogen, NOx is formed by taking nitrogen from the air, as it makes up 78% of the air we breathe. Effective technologies to control NOx emissions in diesel vehicles do exist, but many diesel vehicles still emit NOx at levels above legal limits. This is often due to poor design or defects in emission-control system components.
To address this issue, some countries have implemented scrappage programs to remove older, high-emitting vehicles from the road. Policymakers are also incentivizing the adoption of electric vehicles, which produce zero tailpipe emissions. Additionally, low-emissions zones have been established in cities to reduce the number of high-emitting vehicles and mitigate ambient NOx problems.
Machinery and equipment that burn fuel, such as power plants, also contribute to NOx emissions. These emissions are a result of fuel combustion processes, where nitrogen and oxygen in the atmosphere react to form nitric oxide (NO). This NO then converts to nitrogen dioxide (NO2) within the exhaust system or in the atmosphere. Nitrogen dioxide is a highly reactive gas with a foul smell, appearing as a reddish-brown haze when prevalent in the air.
Power plants and other industrial equipment involving fuel combustion need to implement measures to reduce NOx emissions. One method is flue gas recirculation, which reduces the oxygen content and lowers the temperature in the combustion zone, thereby decreasing NOx emissions. Another technology is Flameless Oxidation (FLOX), which significantly suppresses the formation of thermal NO.
Cleanest Energy Sources: Low-Pollution Power Options
You may want to see also

NOx reacts with volatile organic compounds to form ozone
Nitrogen oxides (NOx) are a group of highly reactive gases, including nitrogen dioxide (NO2), nitric oxide (NO), nitrous oxide, nitrous acid, and nitric acid. These gases are primarily produced through the combustion of fossil fuels, such as coal, oil, and gas, as well as from vehicle, power plant, and off-road equipment emissions caused by fuels burning at high heat.
NOx gases, especially NO2, are harmful pollutants that have negative effects on the respiratory system when inhaled. They also contribute to the formation of secondary pollutants, including particulate matter, acid rain, and toxic chemicals. One of the most significant ways in which NOx contributes to air pollution is through its reaction with volatile organic compounds (VOCs) to form ozone.
VOCs are carbon-containing compounds that easily become vapors or gases. They are emitted from various sources, including the tailpipes of cars, petroleum refineries, and other petroleum industries. When NOx gases and VOCs react in the presence of sunlight and heat, they undergo a complex set of reactions in the troposphere to form ozone (O3). This process can be represented as follows:
VOCs + NOx = Ozone
The formation of ozone through this reaction is a significant environmental concern, as ozone is a secondary pollutant that contributes to ground-level ozone or "bad" ozone, and photochemical smog. Ground-level ozone is harmful to human health and can cause respiratory issues.
The relationship between VOCs, NOx, and ozone concentrations is complex. While initial VOC and NOx concentrations do not directly determine the maximum ozone concentration formed, higher VOC/NOx ratios generally result in ozone concentrations that are less sensitive to VOC concentrations. This means that, at higher VOC/NOx ratios, controlling NOx emissions is more effective in reducing ozone levels.
Overall, the reaction between NOx and VOCs to form ozone is a critical environmental and health issue that requires regulatory attention and effective control strategies to mitigate its harmful impacts.
QI: The Power of Question and Answer Sessions
You may want to see also

NOx contributes to ground-level ozone, smog, and air pollution
Nitrogen oxides (NOx) are a group of highly reactive gases, including nitric oxide (NO) and nitrogen dioxide (NO2). NOx is primarily produced in fuel combustion processes from nitrogen and oxygen in the atmosphere, and emitted from vehicles, machinery, power plants, industrial boilers, refineries, and chemical plants. NOx contributes to ground-level ozone, smog, and air pollution through its reactivity with other chemicals in the air.
Ground-level ozone is a harmful air pollutant and the main ingredient in smog. It is not emitted directly into the air but is formed by chemical reactions between NOx and volatile organic compounds (VOCs) in the presence of sunlight. VOCs are carbon-containing compounds that easily become vapors or gases. These reactions occur when pollutants emitted by cars, power plants, and other sources interact in the atmosphere. Ground-level ozone can have detrimental effects on human health, particularly for children, the elderly, and people with lung diseases such as asthma. It is most likely to reach unhealthy levels on hot, sunny days in urban areas, but can also impact rural regions through wind transportation.
Nitrogen dioxide (NO2) is a significant contributor to NOx pollution and is produced through the combustion of fossil fuels such as coal, oil, and gas. NO2 combines with VOCs in the presence of sunlight and heat to create ozone. This process contributes to the formation of ground-level ozone and the associated negative health impacts. NO2 itself is a harmful gas with a foul smell and can cause respiratory issues when inhaled.
NOx emissions have a substantial impact on air quality and human health. In the UK, emissions from road transport are the largest contributor to NOx pollution, accounting for 33% of total emissions in 2010. The introduction of emission limits for vehicles has helped reduce NOx levels, but more measures are needed to improve air quality and protect public health.
Overall, NOx plays a crucial role in the formation of ground-level ozone, which, along with NOx itself, contributes to smog and air pollution. The interaction of NOx with other chemicals in the atmosphere leads to the creation of harmful pollutants that affect both human health and the environment. Understanding and mitigating NOx emissions are essential steps in improving air quality and safeguarding public well-being.
Pollution's Devastating Impact on Our Planet and Health
You may want to see also
Frequently asked questions
Nitrogen oxides (NOx) are a group of highly reactive gases, including nitrogen dioxide (NO2), nitric oxide (NO), nitrous oxide, nitrous acid, and nitric acid.
Nitrogen oxides are primary pollutants as they are emitted directly into the environment from sources such as vehicles, power plants, and off-road equipment. However, they can also act as precursors to secondary pollutants, forming particulate matter, ozone, and acid rain when they react with other chemicals in the atmosphere.
Nitrogen oxides are formed through the burning of fossil fuels, such as coal, oil, and gas. Specifically, NO is produced during fuel combustion processes and then converted to NO2 within the exhaust system or in the atmosphere.
Nitrogen oxides contribute to air pollution and have harmful effects on human health, particularly the respiratory system. They are a concern for both outdoor and indoor air quality. Additionally, they react with other chemicals to form secondary pollutants, leading to issues such as acid rain.
To reduce nitrogen oxide emissions, improved regulations, technological advancements, and economic changes are necessary. This includes implementing stricter emission standards for vehicles and power plants, promoting the use of alternative energy sources, and enhancing ventilation and air purification systems, especially in densely populated areas.







